Recommendations for Use of Irradiated Blood Components in Canada
Nancy Robitaille, MD; Co-Chair (CCNMT)
Alan Tinmouth, MSc, MD
Andrew Shih, MD
Charles Musuka, MD
Dana Devine, PhD
Douglas Morrison, MD
Vincent Laroche, MD
List of abbreviations
Adverse Transfusion Reaction
British Journal of Haematology
British Society for Haematology
Canadian Blood Services
Canadian Society for Transfusion Medicine
Canadian Standards Association
CBS Provincial Territorial Blood Liaison Committee
Chimeric Antigen Receptor T-cell
Comité consultatif national en médecine transfusionnelle (Québec transfusion medicine advisory committee)
Donor Lymphocyte Infusion
Graft versus Host Disease
Héma-Québec
Hematopoietic Stem Cell Transplantation
Human Leukocyte Antigen
Intraoperative Cell Salvage
Intrauterine Transfusion
National Advisory Committee on Blood and Blood Products
Pathogen Inactivated Technology
Provincial / Territorial Blood Liaison Committee
Public Health Agency of Canada
Québec Hemovigilance System
Red Blood Cell
Serious Hazards of Transfusion
Transfusion Error Surveillance System
Transfusion Transmitted Injuries Surveillance System
Transfusion-Associated Graft-versus-Host Disease
White Blood Cell
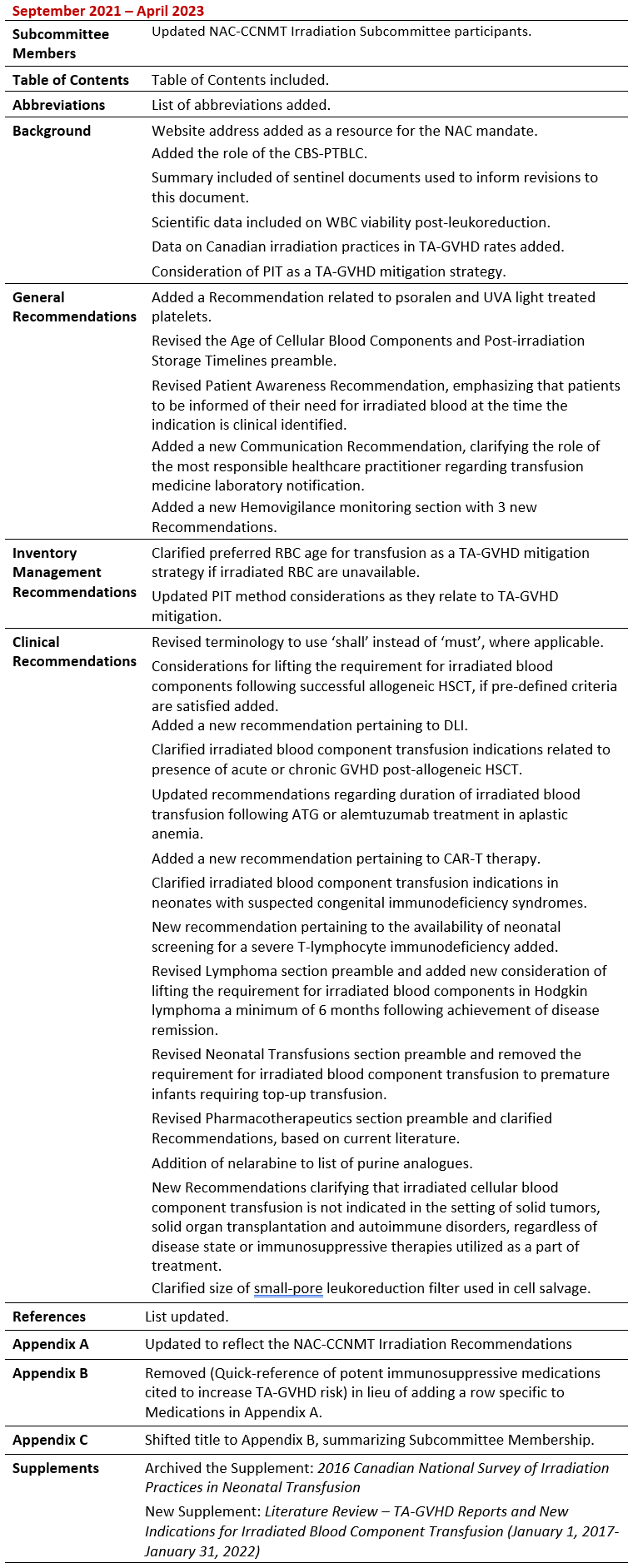
Summary of Revisions
Revision Date
Details

The National Advisory Committee on Blood and Blood Products (NAC) is an interprovincial medical and technical advisory body to the provincial and territorial health ministries and Canadian Blood Services (CBS). The NAC Mandate is found on the nacblood.ca website.
In 2014, the CBS Provincial Territorial Blood Liaison Committee (CBS-PTBLC) requested that the NAC develop recommendations and guidelines for the use of irradiated blood components for Canadian patients. CBS-PTBLC facilitates the work between the Provincial/Territorial (PT) governments (except Québec) and CBS to support the provision of a safe, secure, and affordable national blood supply system and other CBS products and services approved by PT Ministers of Health who serve as CBS’ Corporate Members.
The NAC assembled an Irradiation Working Group to review current standards, published guidelines, and recent literature on the indications for irradiated components and the quality of irradiated red blood cell (RBC) components to facilitate recommendations for best practices. This group worked collaboratively with representatives of Québec’s National Advisory Committee on Transfusion Medicine (CCNMT) who joined the recommendation development process – thus forming the NAC-CCNMT Irradiation Working Group. Consultation with Canadian experts in transfusion medicine was included as a part of this recommendation development process. In addition, draft recommendations were circulated to a number of clinical stakeholder organizations for feedback prior to finalization.
The originally produced document was published in 2017 and was informed by the published guidelines on the use of irradiated blood components by the British Committee for Standards in Haematology, 2010, and the guidelines for prevention of transfusion-associated graft-versus-host disease (TA-GVHD) by the Australian and New Zealand Society of Blood Transfusion, 2011. Published Transfusion Medicine practice standards including the Canadian Standards Association (CSA) Z902-15 standards (2015), Canadian Society for Transfusion Medicine (CSTM) standards (version 4, 2017), and Council of Europe Standards (version 17, 2013); and recent publications on the quality of stored RBC post irradiation and practices involving irradiation of autologous blood collected by intraoperative cell salvage were consulted.
The NAC-CCNMT Recommendations for Use of Irradiated Blood Components in Canada was scheduled for review in 2021. The Irradiation Subcommittee (formerly Working Group) including NAC and CCNMT membership was reconvened to complete this work. The recommendations in this document are informed by the updated guidelines on the use of irradiated blood components by the British Society for Haematology (BSH, 2020) and the Dutch guidelines for prevention of TA-GVHD published in the British Journal of Hematology (BJH, 2021); published Transfusion Medicine practice standards including the CSA Z902-20 standards (2020) and CSTM Standards (version 5, 2021); and a literature review searching for reported cases of TA-GVHD published between January 1, 2017 through January 31, 2022 and updated data on the quality of stored RBC post irradiation. The Supplement includes the literature review summary.
Rationale for the Use of Irradiated Blood
Irradiation of cellular blood components is a well-established intervention for the prevention of transfusion-associated graft-versus-host disease (TA-GVHD). There is a system cost of providing irradiated blood components to patients that includes higher production costs, regulatory compliance, and supply logistics. There is a negative impact of irradiation on the RBC unit quality including membrane damage causing a shortened cellular lifespan and potassium leakage, underscoring the importance of considering RBC age at the time of irradiation and the length of storage post-irradiation (Serrano et al, 2014). Thus, the Irradiation Subcommittee has therefore emphasized the need for conscientious management of hospital inventories to limit the provision of irradiated RBCs to those patients who have specific clinical indications to receive irradiated RBC units. Adherence to this practice would thereby minimize exposure of the general patient population to irradiated RBCs.
Data provided by our national blood operators demonstrate some trends in irradiation practice in Canada over the past 15 years. There had been a national increase in the percentage of irradiated RBCs from 4.5% (2004-05 fiscal year) to 6.7% (2015-16), followed by a modest decrease to 5.9% in 2021-22. There continues to be considerable regional variation, with the percentage irradiated RBC units issued by CBS ranging from 1-20%, depending on the province. The percentage of irradiated RBC units issued by Héma-Québec, in 2021-2022 was 5.6%. The variations in provincial irradiated RBC demand may be attributable to factors including distance of hospitals from the blood distribution site and availability of an in-hospital irradiator, as well as differences in regional transfusion practice patterns.
TA-GVHD is a rare, usually fatal complication of transfusion resulting from the engraftment of transfused, immunocompetent donor lymphocytes and subsequent damage to recipient tissues which are perceived as foreign by the engrafted lymphocytes. Patients at most risk include those who are severely immunocompromised and those who fail to recognize the transfused donor lymphocytes as foreign if human leukocyte antigen (HLA) alleles are haploidentical.
Defining immunocompromised patient groups who should receive irradiated blood has been largely based on observational evidence, case reports, reviews and attempts to predict the degree of immunosuppression. As such, there is a lack of consistency in textbooks, publications, and guidelines as to which patients must receive irradiated blood. Many authors address the lack of clarity by including a category of indications where there is uncertainty regarding the need for irradiated blood.
In view of growing evidence demonstrating the poor quality of post-irradiation stored RBCs, there is a need to be thoughtful about the clinical indications for irradiated blood transfusion and avoid over stocking irradiated RBC units. Without an on-site irradiator, finding a balance of stocking sufficient (but not excess) supply for patients in-need of irradiated blood may be challenging. Concerns regarding post-irradiation component quality and storage do not apply to platelets or granulocyte concentrates due to the short shelf life of these components.
In addition to irradiation as a TA-GVHD mitigation strategy, recent observational and scientific evidence suggests that the pre-storage leukoreduction and length of time since donation may modify recipient TA-GVHD risk. However, pre-storage leukoreduction and increased RBC storage time have not yet conclusively been accepted to have an equivalent efficacy for eliminating TA-GVHD risk.
A systematic review of reported TA-GVHD cases reported that greater than 94% of cases of TA-GVHD occur when the blood is less than 10 days old, with none of the TA-GVHD cases associated with components older than 14 days from collection (Kopolovic et al, 2015). A review of 290 cases referred to the Japanese Red Cross (JRC) from 1992-1999 identified 66 cases of definite TA-GVHD by microsatellite DNA analysis. The oldest blood transfused in this series was 10 days for whole blood, 11 days for RBCs without an additive solution, and 14 days for RBCs with added mannitol, adenine, and phosphate (Uchida et al, 2013). In another report from the JRC, 96% of 51 cases received blood less than 96 hours old (Jawa et al, 2015). Based on this observational data, it appeared that transfusion of blood stored for more than 14 days represented a potential TA-GVHD risk mitigation strategy.
It should be noted that following the 2001 terrorist attacks in New York, a decision was made in the United Kingdom to increase blood stocks. As a result, the mean age of blood issued to hospitals after 2002 went from 8 days to 12-14 days and may have also contributed to reducing TA-GVHD risk (Williamson et al, 2007).
However, a recently published laboratory-based study evaluating the viability and proliferative ability of residual white blood cells (WBC) in leukoreduced RBC units suggested a longer storage duration may be necessary. Filtration of the units depleted WBC, particularly T-lymphocytes, to 0.001% ± 0.003% cells/unit; however, the proliferative activity of these residual WBC remained consistent with pre-filtration WBC. Following storage of the RBCs, viable cells could be detected at 14 days and through day 21 of storage, though their proliferative activity was markedly decreased at day 21 to 0.24% ± 0.41%. The authors concluded that after 21 days, T-cells were sufficiently inactive and may help prevent TA-GVHD when irradiated RBCs are unavailable (Mykhailova et al, 2021).
In addition to the scientific evidence demonstrated by the aforementioned study, the potential risk mitigation afforded by pre-storage leukoreduction is supported by data from the Serious Hazards of Transfusion (SHOT) surveillance system in the United Kingdom. A total of 14 cases of TA-GVHD have been reported to SHOT since 1996, which include only 3 cases since the introduction of universal leukoreduction in 1999. Two of these patients were confirmed to have received leukoreduced components. The first case involved a patient with multiple myeloma, reported in 1999. The second case occurred in 2001 and involved a 14-year-old patient with relapsed acute lymphoblastic leukemia. Donors from potentially implicated units were not recalled for HLA typing. The most recent case, reported in 2012, followed an emergency intrauterine transfusion of non-irradiated, non-leukoreduced maternal RBC. The mother was subsequently found have a homozygous HLA haplotype (Bolton-Maggs, 2012). There have been no further TA-GVHD cases reported to SHOT since 2012 (UK SHOT Annual Reports and Summaries).
A Health Canada – Canada Vigilance search between October 1, 2015 (publication date of the Blood Regulations) and February 28, 2022, yielded 1 report of a serious adverse reaction which included GVHD within its coding; however, review of case details confirmed that TA-GVHD was not involved. Interrogation of the Public Health Agency of Canada (PHAC) Transfusion Transmitted Injuries Surveillance System (TTISS) database between 2010-2020 yielded no reported TA-GVHD cases which were determined to be ‘definitely’, ‘probably’ or ‘possibly’ related to transfusion. As of 2020, over 95% of Canadian hospitals providing transfusion services participate in reporting data to the TTISS database. Since 2010, no cases of TA-GVHD have been reported within the Québec provincial hemovigilance (QHS) system.
Capturing transfusion errors is an important complement to tracking of adverse transfusion reactions. Health Canada’s Blood Regulations do require that transfusing facilities maintain error logs as a quality assurance activity; however, the mechanism or database for error tracking is not specified. The PHAC Transfusion Error Surveillance System (TESS) was initiated in 2005 to monitor errors occurring at any point in the transfusion chain. Within TESS, errors can be tracked using predefined categories and codes. One of these is “PR 03 – Special needs not indicated/incorrect (e.g. auto, irradiated)” may be used to document transfusion of a non-irradiated cellular component to an individual with acceptable indications for an irradiated component. Unfortunately, use of the TESS database is underutilized as a voluntary system, with less than 20% of all transfusing facilities nationwide formally participating in the TESS program. Further, this category may include reported errors where other special transfusion needs are missed. As a result, the current rates of transfusion errors related to administration of non-irradiated units to patients with an existing indication are unknown.
Pathogen inactivation technologies (PIT) for pooled and apheresis platelet components will actively be implemented by CBS production sites over the next several years. In January 2022, CBS began offering psoralen treated platelets, utilizing the Cerus INTERCEPT® DS pathogen reduction technology. According to the INTERCEPT® package insert, platelets manufactured by this process do not require irradiation as the PIT processing also inactivates residual donor T-cells. Widespread use of this PIT for platelet transfusion in Europe has not led to any increase in TA-GVHD cases to date (Cid et al. 2017).
Several PIT systems are currently available worldwide, though not all are Health Canada approved. A careful review of the manufacturer recommendations will be required as PIT systems are introduced in Canada to confirm whether the PIT confers protection against TA-GVHD, or whether the components require irradiation for this risk mitigation. Some evidence even suggests that PIT may be equivalent or even superior to gamma irradiation for mitigating the risk of TA-GVHD, and that it should be universally applied (Cid et al. 2017). Clinical research involving PIT is ongoing and this data continues to evolve.
Document Scope and Guide
Upon review of the best-available information, the NAC-CCNMT Irradiation Subcommittee has updated its original recommendations document to help Canadian clinicians determine which patients should receive irradiated components and define the age of blood components at the time of irradiation and the length of storage post-irradiation. It is assumed that laboratories which irradiate blood components do so in accordance with published Canadian transfusion medicine standards and regulations.
Special annotations following each recommendation have been included to inform the reader of the recommendation reference:
- An asterisk (*), to indicate a verbatim statement from the referenced guideline.
- A level of evidence description for the recommendation may be included from the original guideline document, if available.
- Recommendation statements without a reference are considered best practice statements by NAC-CCNMT and the Irradiation Subcommittee, based upon published guidelines or literature.
Recommendations pertaining to the RBC age and the length of storage post-irradiation are consistent with the CSA Z902-20 standards (2020) and the CSTM Standards (version 5, 2021).
In defining patients who should receive irradiated cellular components, the Subcommittee supports the majority of published BSH 2020 guidelines from the United Kingdom and BJH 2021 guidelines from the Netherlands. Differences between these two evidence-based guidelines are summarized in a published commentary, which highlights use of the same evidence to arrive at some different conclusions (Bolton-Maggs, 2021).
Unless otherwise specified, recommendations made within this document are applicable to both adult and pediatric populations.
The NAC-CCNMT Irradiation Subcommittee supports the following general recommendations pertaining to the type of blood component that requires irradiation, information sharing and communications of a patient’s requirement for irradiated blood components, and hemovigilance.
1. Blood components that should be irradiated
- Recommendation: For at-risk patients, all RBC, platelet, and granulocyte concentrates should be irradiated, except cryopreserved RBC after deglycerolization. It is not necessary to irradiate fresh frozen plasma, cryoprecipitate, or fractionated plasma products.
(BSH 2020, Grade 1 recommendation; level B evidence)* - Recommendation: All transfusions from first- or second-degree relatives shall be irradiated, even if the patient is immunocompetent.
- Recommendation: All HLA-selected (matched) platelets shall be irradiated, even if the patient is immunocompetent.
- Recommendation: All granulocyte components shall be irradiated before issue.
- Recommendation: Pathogen inactivated cellular components treated with psoralen and UVA light should not be irradiated.
Please see section 6.C. below for management of inventory in the setting of emergency transfusion where irradiated blood components are not available.
2. Age of cellular blood components and post-irradiation storage timelines
Recent evidence has shown that the age of RBCs at the time of irradiation is important. CBS has tested over 900 RBC units at different stages of storage and irradiation combinations and demonstrated that RBCs irradiated late in the storage period or held for long periods of time have higher hemolysis levels and potassium levels than non-irradiated RBCs, and decreased post-transfusion recovery (Serrano et al, 2014). These finding have implications for patient safety as well as hospital inventory management practices.
The most recent revision of the CSA Z902-20, 2020 standards pertaining to irradiated RBC storage have become more stringent with their timelines. The CSTM Standards version 5, 2021 are in alignment with the current CSA Standards. To enable a maximal 14-day RBC storage period within hospitals, both CBS and HQ have an internal practice to irradiate RBCs within 14 days of collection. Expiry dates on the RBCs ensure compliance with the CSA 2020 Standards.
In general, little attention is paid to the irradiation of platelets or granulocytes. Irradiation causes increased vesiculation of platelets and may shorten their in vivo survival, but it is not clear if this is clinically relevant in platelet transfusion recipients. Pathogen inactivation technologies principally use ultraviolet irradiation, but this process has also been shown to damage platelets to the point that they remain in the circulation for a shorter period of time and are more activated than untreated platelets at the time of infusion (McCullough et al, 2004; Pineda et al, 2006; Snyder et al, 2005; van Rhenen et al, 2003; van Rhenen et al, 2004). This may result in more frequent transfusion for some patients (Garban et al, 2018).
- Recommendation: Red cell components may be irradiated up to 28 days after collection. Irradiated cells must be transfused as soon as possible, but no later than 14 days after irradiation, and in any case, no later than 28 days after collection.
(Canadian Standarts Association, Z902-20, 2020)* - Recommendation: Platelets can be irradiated at any stage during storage and can thereafter be stored up to their normal shelf life after collection.
(BSH 2020, Grade 1 recommendation; level A evidence)*
3. Patient awareness
- Recommendation: Patients at risk of TA-GVHD should be made aware of their need for irradiated blood components. It is the responsibility of the most responsible health care practitioner to inform patients at risk of TA-GVHD of their need for irradiated blood components at the time an indication for irradiated blood transfusion is identified.
4. Communication
- Recommendation: Where any patient has an appropriate indication to receive irradiated blood transfusion, it is the responsibility of the most responsible healthcare practitioner to communicate the indication for irradiated blood transfusion to the transfusion medicine laboratory.
- Recommendation: To ensure consistency of patient care across jurisdictions, particularly between hospital facilities that participate in the shared care of patients, a communications process between clinicians and the transfusion medicine laboratory facilitating sharing details of special transfusion requirements should be implemented and maintained as a best practice policy. In an ideal setting, an electronic automatic notification identifying pharmaceutical history or indication for irradiated blood to the transfusion medicine laboratory would be in place.
5. Hemovigilance monitoring
- Recommndation: Hemovigilance data should be maintained by the Transfusion Medicine service on patients with indications for irradiated blood component transfusion, but who were transfused with cellular blood components which were not irradiated (or considered irradiated equivalent, i.e. pathogen reduced, or stored for at least 14 days and transfused in an emergency setting). Where this error has occurred, documentation of the circumstances should be recorded in the patient healthcare record and within jurisdictional safety reporting systems.
- Recommendation: Where a patient with indications for irradiated cellular component transfusion is identified to have received a non-irradiated blood component transfusion unintentionally or out of necessity (i.e. life-threatening bleeding), a discussion regarding the patient risk of TA-GVHD as assessed at the time the incident is discovered should take place between the transfusion medicine physician and the most responsible healthcare provider. If it the TA-GVHD risk is determined to be significant, the physician who placed the order for the implicated blood transfused shall be responsible for communicating the incident to the patient and for ensuring clinical monitoring for any evidence of TA-GVHD over the next 6 weeks.
Investigation for TA-GVHD should be initiated in a patient who presents with a clinical syndrome, 1 to 6 weeks following transfusion, characterised by a constellation of signs and symptoms which may include an erythematous maculopapular rash, fever, hepatosplenomegaly, liver dysfunction, pancytopenia, or marrow aplasia. Investigation should include tissue biopsy (skin or liver) and molecular chimerism studies on peripheral blood or tissue. The inability to establish evidence of chimerism does not preclude a diagnosis, however it is important for the assessment of imputability. Canadian hemovigilance system definitions include those found in the Public Health Agency of Canada - TTISS Users’ Manual or the INSPQ Québec Hemovigilance System Guide de déclaration des événements indésirables associés à la transfusion de produits sanguins (available in French only). As Canadian resource definitions of TA-GVHD currently do not include transfusion imputability categories, consultation of details within the United States National Health And Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol may be considered.
- Recommendation: All cases of suspected or confirmed TA-GVHD shall be reported to the blood operator as soon as possible, including prior to completion of the investigation.
If the implicated blood component was provided by the blood operator, then the case must be reported to the blood operator, who completes a report to Health Canada – Canada Vigilance. If the blood component was manipulated in any way by a hospital facility, then a report must be submitted by the facility directly to Health Canada – Canada Vigilance.
As stated, prolonged storage of pre-irradiated units is associated with high potassium levels, in vitro hemolysis and decreased post-transfusion recovery (Serrano et al, 2014). Maintaining large inventories of irradiated RBCs results in potentially harmful transfusion of irradiated RBCs to patients who do not require irradiated cellular blood components.
Proactive inventory management must take into consideration the perceived risk of TA-GVHD in the local patient population, the risks of transfusing irradiated RBCs to patients who do not require irradiated cellular components and the logistics of providing irradiated components for elective transfusions. Irradiation of RBCs should therefore occur as near-to as possible the time of transfusion.
6. Irradiated blood component availability
- Recommendation: For elective transfusions reliance on a regional hub site for on-demand irradiation or limited pre-irradiated stock is recommended.
- Recommendation: Overstocking of pre-irradiated units for emergency transfusion is not recommended. If storage of pre-irradiated inventory is absolutely necessary, then RBCs that have been irradiated within 14 days of collection should be obtained, if possible.
As described above, observational evidence from UK SHOT data (Williamson et al, 2007), a systematic review of 348 cases of TA-GVHD (Kopolovic et al, 2015) and three reviews of Japanese Red Cross data had suggested a risk mitigating effect of universal pre-storage leukoreduction and the transfusion of RBCs greater than 14 days from donation (Uchida et al, 2013; Jawa et al, 2015). However, a more recent scientific study concluded that after 21 days of hypothermic RBC storage, T-cells were sufficiently inactive and may help prevent TA-GVHD when irradiated RBCs are unavailable (Mykhailova et al, 2021).
- Recommendation: In the event of emergency transfusion in the absence of on-site irradiation or pre-storage irradiated inventory, pre-storage leukoreduced RBCs that have been stored for at least 14 days, but preferably more than 21 days, should be provided to patients with an indication for irradiated blood transfusion.
- Recommendation: Where there is concern about the immunosuppressive potency of new drugs and uncertainty about the risk of TA-GVHD, in the absence of on-site irradiation or pre-storage irradiated inventory, pre-storage leukoreduced RBCs that have been stored for at least 14 days, but preferably more than 21 days, should be provided.
- Recommendation: Pathogen inactivation or reduction technologies may be considered an alternative or equivalent to irradiation as TA-GVHD mitigation strategies. The manufacturer recommendations shall be consulted to determine whether irradiation of the component following pathogen inactivation or reduction treatment application is necessary.
To arrive at the following clinical recommendations the NAC-CCNMT Irradiation Subcommittee has drawn primarily upon the BSH 2020 guidelines from the United Kingdom and BJH 2021 guidelines from the Netherlands, which have included current evidence supporting their recommendations.
For clinical conditions listed, the patient medication history must be taken into consideration by the most responsible healthcare practitioner requesting blood transfusion, as a therapy received may necessitate irradiated cellular component use. The indication for irradiated blood component transfusion is a clinical decision and requires clear communication from the ordering practitioner to the Transfusion Medicine service. The Transfusion Medicine service must ensure appropriateness of irradiated blood issue and have a policy listing acceptable indications for irradiated blood transfusion. Upon identifying an inappropriate request for irradiated blood transfusion, the Transfusion Medicine service should have a mechanism for engaging in follow-up communications with ordering practitioner to review the request.
The indication for transfusion of irradiated blood components should be reviewed regularly (e.g. annually) by healthcare practitioners in patient who have acquired disease or treatment related indications for irradiated blood transfusion, as in some instances, lifting the indication for irradiated blood transfusion may be considered. If the most responsible healthcare practitioner determines that discontinuing transfusion of irradiated blood components is appropriate, this change must be clearly communicated with the Transfusion Medicine service, in accordance with local policy.
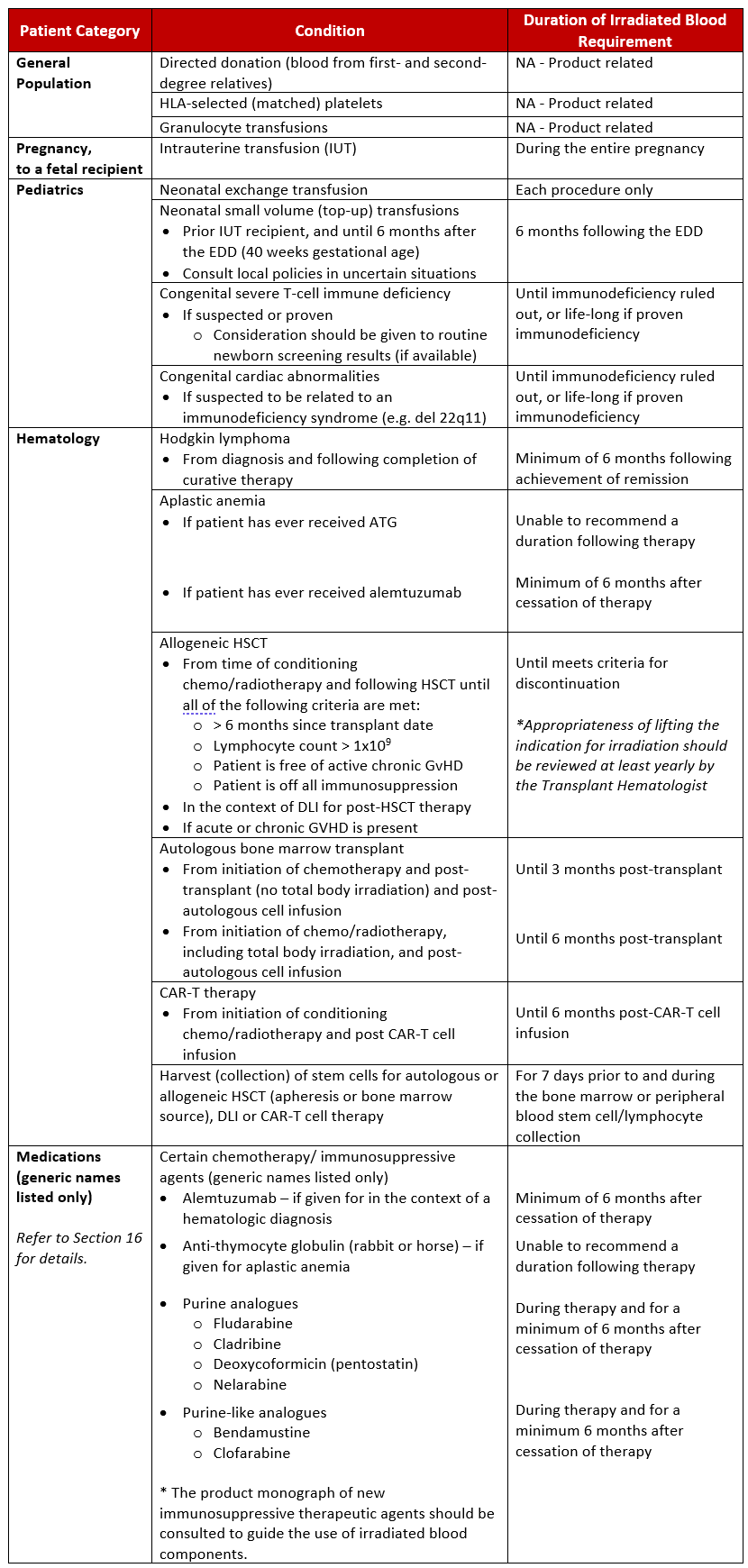
Appendix A provides a quick reference of the clinical indications for transfusion of irradiated blood components.
7. Acute leukemia
There is no definitive evidence to support the need for irradiated blood transfusion to patients with a diagnosis of acute leukemia (any type) in adult and pediatric patients, in the absence of identified TA-GVHD risk factors. Chemotherapy regimens for acute leukemia typically do not include pharmacotherapeutic agents known to be risk factors for TA-GVHD development. However, individual treatment protocols should be reviewed to ensure that there is no underlying indication for irradiated blood transfusion (see Section 16 below).
- Recommendation: Routine irradiation of RBCs or platelets for adults and children with acute leukemia is not recommended, except for HLA-selected platelets, transfusion of granulocytes, donations from first- or second-degree relatives, or in the context of a pre-existing clinical indication or due to current or previous treatment with a therapy with an indication for irradiated blood components.
8. Allogeneic hematopoietic stem cell transplantation
Published clinical practice guidelines agree with the provision of irradiated blood components to allogeneic bone marrow transplant recipients from the time conditioning chemotherapy is initiated. The NAC-CCNMT recommendations align with the BSH 2020 guidelines; however, our Irradiation Subcommittee has maintained a more stringent approach with the requirement for irradiated cellular components from the time of conditioning (shall terminology), rather than the option thereof (should terminology) within the BSH 2020 publication.
However, there is less certainty as to the definition of when transfusion of irradiated blood components can stop post-transplant. The NAC-CCNMT Irradiation Subcommittee agrees with the suggested discontinuation criteria listed in the BSH 2020 publication to guide clinician decision. Due to the paucity of evidence, the decision to discontinue transfusion of irradiated blood components should remain at the discretion of the transplant physician team.
- Recommendation: All adult and pediatric recipients of allogeneic hematopoietic stem cell transplantation (HSCT) shall receive irradiated blood components from the time of initiation of conditioning chemo/radiotherapy. The recommendation applies for all conditions where HSCT is indicated regardless of the underlying diagnosis.
- Recommendation: Irradiated blood components should be continued while the patient continues to receive graft-versus-host disease (GVHD) prophylaxis.
- Recommendation: The indication for ongoing transfusion of irradiated blood components should be reviewed at least yearly. The transplant physician may consider lifting the requirement for irradiated blood components if all of the following criteria are met:
- More the 6 months have elapsed since the transplant date;
- The lymphocyte count is more than 1 x 109/L;
- There is no evidence of active GVHD; and,
- All immunosuppression has been discontinued.
- Recommendation: Irradiated blood components should be continued in patients requiring donor lymphocyte infusion (DLI) in the context of their post-allogeneic transplant therapy. The duration of irradiated blood transfusion post-DLI is contingent upon clinical factors related to engraftment post HSCT, the presence of acute or chronic GVHD and ongoing immunosuppressive therapy.
- Recommendation: If acute or chronic GVHD is present and immunosuppressive treatment is required, or appears after the irradiation indication has been removed and immunosuppressive treatment is reinstated, irradiated blood components should be given indefinitely.
- Recommendation: Allogeneic blood transfused to bone marrow harvest and peripheral blood stem cell donors of all ages within 7 days prior to or during the harvest and/or peripheral collection should also be irradiated.
9. Aplastic anemia
- Recommendation: Patients with aplastic anemia undergoing treatment with anti-thymocyte globulin (ATG) or alemtuzumab should receive irradiated blood components.
(BSH 2020, Grade 2 recommendation; level C evidence)* - Recommendation: Due to the paucity of evidence, a firm recommendation as to how long irradiated blood components should continue to be used after ATG administration in patents with aplastic anemia cannot be made.
- Recommendation: Patients who have received alemtuzumab (anti-CD52) therapy in the context of aplastic anemia shall receive irradiated blood components for a minimum of 6 months after cessation of therapy.
- Recommendation: In the absence of indications necessitating irradiated component transfusion, patients with aplastic anemia do not otherwise require transfusion of irradiated RBCs and platelets.
See Section 16. for additional information about indications for irradiation related to use of ATG and alemtuzumab use in the context of hematologic diagnoses.
10. Autologous hematopoietic stem cell transplantation
- Recommendation: Patients undergoing bone marrow or peripheral blood stem cell ‘harvesting’ for future autologous re-infusion should receive irradiated cellular blood components during and for 7 days before the bone marrow/stem cell harvest to prevent the collection of viable allogeneic T-lymphocytes, which can potentially withstand cryopreservation.
(BSH 2020, Grade 1 recommendation; level C evidence)* - Recommendation: All patients undergoing autologous bone marrow transplant or peripheral blood stem cell transplant should receive irradiated cellular components from initiation of conditioning chemo/radiotherapy until 3 months post-transplant (6 months if total body irradiation was used in conditioning).
(BSH 2020, Grade 1 recommendation; level C evidence)*
11. Chimeric antigen receptor T-cell (CAR-T) therapy
- Recommendation: Patients undergoing peripheral blood lymphocyte collections for future CAR-T cell re-infusion should receive irradiated cellular components for 7 days prior to and during the harvest, to prevent the collection of viable allogeneic T-lymphocytes. Irradiated blood components should continue to be used until 6 months following CAR-T cell infusion (due to use of purine analogue based conditioning) unless other pharmacotherapies, disease or previous treatment determine a different duration of irradiated blood transfusion.
12. Immunodeficiency states, acquired
- Recommendation: There is no indication for irradiation of cellular blood components for infants or children with temporary defects of T-lymphocyte function as a result of a viral infection. There is also no indication for routine irradiation of cellular blood components for adults or children who are HIV antibody positive or who have acquired immune deficiency syndrome (AIDS).
(BSH 2020, Grade 1 recommendation; level B evidence)*
13. Immunodeficiency states, congenital
- Recommendation: All severe T-lymphocyte immunodeficiency syndromes with significant qualitative or quantitative T-lymphocyte deficiency should be considered as indications for irradiation of cellular blood components.
(BSH 2020, Grade 1 recommendation; level B evidence)* - Recommendation: Once a diagnosis of severe T-lymphocyte immunodeficiency has been suspected, irradiated components should be given while further diagnostic tests are being undertaken. A clinical immunologist should be consulted for advice in cases where there is uncertainty.
(BSH 2020, Grade 1 recommendation; level C evidence)* - Recommendation: If possible, neonates and infants with suspected immunodeficiency syndromes should undergo investigation for T-cell immune deficiency prior to cardiac surgery. If it is not possible for results to be reported prior to surgery, irradiated cellular blood components should be given until immunological investigations have been completed.
- Recommendation: Adults and children aged greater than 2 years without a significant history of infections referred for elective cardiac surgery for problems associated with DiGeorge syndrome, such as aortic arch anomalies and pulmonary artery stenosis, or in whom DiGeorge anomaly is suspected, do not need to receive irradiated cellular blood components, unless there is a significant history consistent with severe T-lymphocyte-associated immunodeficiency, as the risk of TA-GVHD is extremely low.
(BSH 2020, Grade 2 recommendation; level C evidence)* - Recommendation: In centers where neonatal screening for a severe T-lymphocyte immune deficiency is available, a communication mechanism should be established to ensure screening test and confirmatory test result communication with the Transfusion Medicine service to ensure appropriate use of irradiated blood components in infants.
14. Lymphoma
Available literature suggests that the risk of TA-GVHD in Hodgkin lymphoma is greater than in those with non-Hodgkin lymphoma. This risk apparently is unrelated to Hodgkin lymphoma stage or treatment modality, with reported cases of late TA-GVHD in these patients (Spitzer et al., 1990). Though updated hemovigilance data suggests an extremely low incidence of TA-GVHD or no TA-GVHD identified in the some hemovigilance systems despite patients receiving non-irradiated components in error, BSH 2020 guidelines and BJH 2021 guidelines from the Netherlands conflict in their recommendations (Bolton-Maggs, 2021).
Expert opinion varies and there is a lack of evidence justifying an indefinite indication for irradiation in the setting of Hodgkin lymphoma.
- Recommendation: All adults and children with Hodgkin lymphoma shall receive irradiated RBCs and platelets from the time of diagnosis (regardless of stage) and for a minimum of 6 months following achievement of remission.
Due to the paucity of evidence, the decision to discontinue transfusion of irradiated blood components in this setting remains at the discretion of the treating physician.
The diagnosis of non-Hodgkin lymphoma on its own is not an indication for irradiated blood component transfusion. However, the use of certain therapies may necessitate the use of irradiated blood component transfusion. (See Section 16 below for further details).
15. Neonatal Transfusions
In 2016, a survey organized by the NAC was conducted to better understand current practices in Canada regarding the use of irradiated blood components specifically for neonatal populations, which was used to inform initial recommendations related to neonatal irradiated blood transfusion. Subsequently, publication of the BSH 2020 and BJH 2021 guidelines and related literature have served as sentinel resources for revision of recommendations in this section.
The NAC-CCNMT Working Group recommendations for neonatal transfusions are as follows:
Intra-Uterine Transfusions (IUT):
- Recommendation: All components for IUT must be irradiated. To minimize the effect of potassium load, RBCs for IUT must be as fresh as possible, and must be transfused within 24 hours of irradiation.
- Recommendation: Irradiated cellular components are recommended for neonates who have received an IUT, in which case irradiated components should be administered until 6 months after the expected delivery date (40 weeks gestation).
Neonatal Exchange Transfusion:
- Recommendation: It is essential to irradiate blood for neonatal exchange transfusion if there has been a previous IUT or if the donation comes from a first- or second-degree relative.
- Recommendation: For other neonatal exchange transfusion cases, irradiation is recommended provided this does not unduly delay transfusion.
- Recommendation: RBCs for neonatal exchange transfusion should be as fresh as possible and must be transfused within 24 hours of irradiation.
If maternal blood is the only compatible blood for a neonate, it MUST be irradiated as a directed haploidentical donation (see Section 1, Recommendation B).
Neonatal Small-volume (Top-up) Transfusions:
For small volume transfusions in neonates, the NAC-CCNMT Irradiation Subcommittee recognizes that there is a paucity of data to firmly guide transfusion practice. Both the BSH 2020 guidelines from the United Kingdom and the BJH 2021 guidelines from the Netherlands do not recommend transfusion of irradiated RBCs for small-volume transfusion for very low birth weight infants, defined by the World Health Organization as less than 1500 g at birth. The Subcommittee endorses this recommendation based on the fact that the United Kingdom has not performed RBC irradiation for small-volume transfusion to very low birth weight infants since 1996 (all products are leukoreduced), except following IUT and for congenital immunodeficiency with significant quantitative or qualitative T-lymphocyte deficiency, with no cases of related TA-GVHD reported by their robust hemovigilance system (UK SHOT). Furthermore, historical cases of neonatal TA-GVHD occurred in the pre-leukoreduction era.
The NAC-CCNMT Irradiation Subcommittee recognizes that this recommendation is a paradigm shift and that some institutions may decide to continue to provide irradiated blood components for neonatal top-up transfusions to very low birthweight infants.
- Recommendation: Premature babies defined as less than 1500 g birth weight and/or less than 32 weeks do not routinely require irradiated RBCs or platelets except for congenital immunodeficiency and following IUT.
- Recommendation: Where the patient is at particular risk of hyperkalemia, it is recommended that RBCs be transfused within 24 hours of irradiation. RBCs stored for greater than 24 hours from irradiation must at least undergo centrifugation and supernatant plasma removal prior to transfusion.
Ideally, facilities with Level 3 neonatal intensive care units should have an on-site irradiator to facilitate irradiation of RBC aliquots at the time of issue.
For hospitals without an on-site irradiator, it may be necessary to maintain a small stock of RBC units irradiated by the supplier or a regional hub hospital. In this setting, the practice of sharing a unit amongst more than one neonate should be considered as a mechanism of optimally utilizing a freshly irradiated RBC unit and minimizing the length of storage post irradiation. For a neonate requiring repeat RBC transfusion, this practice may increase donor exposure, but should facilitate a regular inventory rotation (e.g. weekly) of a shared pre-storage irradiated RBC unit, and thereby mitigate the risks associated with component quality.
In all cases of neonatal small volume transfusion, aliquots must be less than 14 days post-irradiation and no more than 28 days since donation as specified in the current CSA Z902-20 Standards. Aliquots more than 24 hours from irradiation must have at least undergone centrifugation and supernatant plasma removal. A recent study has demonstrated that supernatant reduction by centrifugation is preferable to gravity settling (Serrano et al, 2017). The final hematocrit should not exceed 0.80 L/L (CSA Z902-20 Standards, 2020). All manipulation should be performed as near-to as possible to the time of RBCs release for transfusion.
Emergency Transfusions:
- Recommendation: For emergency transfusions of unmatched group O RBCs for neonatal resuscitation due to obstetrical complications and/or accidents, irradiated cellular components are not required.
Congenital Cardiac Abnormalities:
The presence of a congenital cardiac abnormality identified in a neonate or infant may raise the suspicion of chromosome 22q11 deletion syndrome, commonly associated with a congenital T-cell immunodeficiency. Cardiac abnormalities most frequently associated with chromosome 22q11 deletions include: Tetralogy of Fallot, ventricular septal defect, interrupted aortic arch, combined pulmonary atresia and ventricular septal defect, and truncus arteriosus (Ryan et al, 1997).
- Recommendation: Irradiated RBCs or platelets are not required for infants undergoing cardiac surgery, unless clinical or laboratory features suggest a coexisting T-lymphocyte immunodeficiency syndrome.
- Recommendation: All neonates with complex cardiac abnormalities should receive irradiated cellular components until a congenital T-cell immune deficiency disorder is excluded by diagnostic testing. If a congenital immune deficiency disorder is confirmed, irradiated cellular components should be provided for life. (see Section 13 - Immunodeficiency states, congenital)
16. Pharmacotherapeutics: Purine analogue drugs and other potent T-cell targeted immunosuppressive therapies for hematologic indications
Treatment with some purine analogues and similar therapies produce severe and durable lymphopenia. Case reports of TA-GVHD have been reported following treatment with the purine analogues fludarabine and cladribine. No cases of TA-GVHD secondary to purine analogues have been reported in the UK SHOT hemovigilance data since the introduction of universal leukoreduction. The updated BSH 2020 guidelines from the United Kingdom continue to recommend indefinite provision of irradiated blood components to recipients of purine analogues. However, the BJH 2021 guidelines from the Netherlands suggest irradiated components should be provided for at least 6 months after cessation of therapy with purine analogues, followed by the consideration of removing the indication for irradiation.
TA-GVHD risk has not been confirmed with purine-like analogues, but often irradiated blood components are provided given that their mechanism of action is similar to that of purine analogues.
The NAC-CCNMT Irradiation Subcommittee recognizes that there is limited data to guide a firm recommendation regarding provision of irradiated blood to recipients of purine analogues and purine-like analogs. We agree that consideration may be given to lifting the indication for irradiated cellular blood component transfusion 6 months after cessation of these therapies.
The decision to provide irradiated blood for patients on specific immunosuppressive agents should be made by the patient’s most responsible healthcare provider, with consideration given to the perceived benefits and risks of irradiated blood transfusion and availability of irradiated blood. Where there is uncertainty or concern regarding the immunosuppressive potency of a particular agent, particularly in the context of therapies described to directly impair T-cell function in the context of treatment for a hematologic malignancy, discussion with a Transfusion Medicine expert is encouraged.
- Recommendation: All patients treated with purine analogue drugs (fludarabine, cladribine, deoxycoformicin (pentostatin), and nelarabine) shall receive irradiated blood components for a minimum of 6 months after cessation of therapy.
- Recommendation: The risk with other purine antagonists and new or related purine-like agents (e.g. bendamustine and clofarabine) is unclear. As these agents have a similar mode of action to purine analogues, patients should receive irradiated blood components for a minimum of 6 months after cessation of therapy.
Irradiated blood transfusion is not required for patients treated with 6-mercaptopurine (6-MP) and azathioprine. These medications are not considered to have the same immunosuppressive potency as the above-listed chemotherapeutic agents, and based on available literature, have not been identified to be associated with an increased TA-GVHD risk.
Potent targeted T-cell antibody therapies as immunosuppressant agents in the context of hematologic malignancies have been associated with an increased risk of TA-GVHD. However, recommendations regarding the use of irradiated blood components in the context of their use in other conditions differs (see Section 17 below for details).
- Recommendation: Patients who have received alemtuzumab (anti-CD52) therapy in the context of aplastic anemia or hematologic malignancies shall receive irradiated blood components for a minimum of 6 months after cessation of therapy.
ATG is a potent immunosuppressive agent which is available as a derivative of both horse and rabbit sources and used in a number of clinical contexts. There is expert consensus that irradiated blood components should be provided to patients with severe aplastic anemia who receive ATG (see Section 9 above).
- Recommendation: Patients who have received ATG therapy in the context of aplastic anemia or hematologic malignancies should receive irradiated blood components.
- Recommendation: Due to the paucity of evidence, a firm recommendation as to how long irradiated blood components should continue to be used after ATG administration in patents with aplastic anemia cannot be made.
There is no evidence of increased TA-GVHD risk in when ATG is used for conditioning in solid organ transplant recipients conditioned with ATG, and as a result, current guidelines for solid organ transplantation do not cite a specific requirement for transfusion of irradiated blood in this population (KDGIO Transplant Work Group, 2009; see Section 17 below). The risk of TA-GVHD in patients undergoing reduced-intensity conditioning with ATG alone for allogeneic hematopoietic stem cell transplantation is unknown.
It is difficult to provide a clear recommendation for or against the use of irradiated blood in the context of all available immunosuppressive and biologic therapies in the absence of published evidence for specific agents in specific clinical contexts. This is particularly the case for new pharmacotherapeutic agents.
- Recommendation: Where a directive is not otherwise provided by published clinical recommendations, the product monograph of new immunosuppressive therapeutic agents should be consulted by the prescribing physician to guide the use of irradiated blood component transfusion.
B-cell targeted therapies in the context of hematologic malignancies (e.g. rituximab) do not have a requirement for irradiated blood component transfusion.
17. Solid tumors, solid organ transplantation, autoimmune disorders, acquired immunodeficiency
- Recommendation: It is not necessary to irradiate blood components for patients with solid tumors, unless use of irradiated blood components is indicated for a different reason (e.g. underlying diagnosis, type of component or previous treatment).
- Recommendation: Use of irradiated cellular blood components is not indicated following treatment with alemtuzumab using the schedule currently recommended for Multiple Sclerosis or vasculitis.
(BSH 2020, Grade 1 recommendation; level B evidence)* - Recommendation: Use of irradiated cellular blood components is not indicated for patients undergoing solid organ transplantation who have received alemtuzumab or ATG as induction therapy or for treatment of graft rejection.
(BSH 2020, Grade 1 recommendation; level B evidence)* - Recommendation: Treatment of patients with rituximab is not an indication for use of irradiated cellular blood components unless this is indicated for a different reason (underlying diagnosis, type of component or previous treatment).
(BSH 2020, Grade 1 recommendation; level B evidence)*
18. Routine surgery and intraoperative cell salvage
Intraoperative autologous red blood cell salvage techniques are important to reduce the rate and associated risks of allogeneic blood transfusion during major surgery. In patients with cancer, the use of intraoperative cell salvage (ICS) has been controversial due to the theoretical risk of metastasis propagation from reinfusion of autologous blood contaminated with metastatic cells. However, a recent meta-analysis of 10 studies showed no significant difference in cancer recurrence rates between patients who received ICS compared to those who did not during their surgeries (Waters et al, 2012). A systematic review evaluating the use of ICS in metastatic spine tumor surgery (Kumar et al, 2014) also did not identify a greater risk of tumor dissemination or metastasis in patients who received reinfusion of autologous ICS blood, though a caution was noted in situations of tumor rupture.
The use of either gamma irradiation or a small-pore (40 um or less) microfiber leukocyte reduction filter have been identified to be effective strategies to reduce the risk of metastatic cell transmission from ICS blood in patients with malignancy undergoing surgery (Trudeau et al, 2012). Leukoreduction filters are more accessible than on-site hospital irradiators and do not impose damage to the RBC membrane, making their use a much more common in practice. The risks and benefits of intraoperative cell salvage in the setting of surgery in patients with suspected or known malignancy must be weighed by the perioperative team and discussed together with the patient prior to use.
- Recommendation: In patients with malignancy undergoing surgery, it is not necessary to irradiate autologous blood collected by intraoperative cell salvage if a small-pore (40 um or less) microfiber leukocyte reduction filter is used prior to blood reinfusion.
- Recommendation: It is not necessary to irradiate blood components for patients undergoing routine surgery, unless use of irradiated blood components is indicated for a different reason (underlying diagnosis, type of component or previous treatment).
References
Bolton-Maggs PHB. Chair of the Working Expert Group & Writing Group, on behalf of the SHOT Steering Group, 2012. https://www.shotuk.org/wp-content/uploads/SHOT-Annual-Report-20121.pdf
Bolton-Maggs PHB. Chair of the Working Expert Group & Writing Group, on behalf of the SHOT Steering Group, 2016. https://www.shotuk.org/wp-content/uploads/SHOT-Report-2016_web_7th-July…
Bolton-Maggs PHB. Guidelines: the same evidence but different conclusions – relaxation of indications for irradiation of cellular blood components? Br J Haematol. 195:657-9, 2021.
Canadian Society for Transfusion Medicine (CSTM) Standards for Hospital Transfusion Services, Version 5, December 2021.
Canadian Standards Association (CSA) Blood and Blood Components. Z902-20. Mississauga ON: Canadian Standards Association, 2020.
Cid J. Prevention of transfusion-associated graft-versus-host disease with pathogen-reduced platelets with amotosalen and ultraviolet A light: a review. Vox Sanguinis. 112:607-13, 2017.
Garban F, Guyard A, Labussière H, Bulabois CE, Marchand T, Mounier C, Caillot, D, Bay JO, Coiteux V, Schmidt-Tanguy A, Le Niger C, Robin C, Ladaique P, Lapusan S, Deconinck E, Rolland C, Foote AM, François A, Jacquot C, Tardivel R, Tiberghien P, Bosson JL; Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process (EFFIPAP) Study Group. Comparison of the Hemostatic
Efficacy of Pathogen-Reduced Platelets vs Untreated Platelets in Patients with Thrombocytopenia and Malignant Hematologic Diseases: A Randomized Clinical Trial. JAMA Oncology. 4: 468-475, 2018.
Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. British Journal of Haematology. 191: 704-24, 2020.
Jawa RS, Young DH, Stothert JC, Kulaylat MN and Landmark JD. Transfusion-associated graft versus host disease in the immunocompetent patient: an ongoing problem. Journal of Intensive Care Medicine. 30: 123-130, 2015.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation. Suppl 3: S1-155, Nov 2009.
Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS and Callum J. A systematic review of transfusion-associated graft versus-host disease. Blood. 126: 406-414, 2015.
Levitt J, ed. Standards for blood banks and transfusion services. 29th ed. Bethesda, MD: AABB, 2014.
Kumar N, Chen Y, Zaw AS, Nayak D, Ahmed Q, Soong R, and Wong HK. Use of intraoperative cell-salvage for autologous blood transfusion in metastatic spine tumor surgery: a systematic review. Lancet Oncology. 15: e33-41, 2014.
McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F 4th, Davis K, Lin JS, Metzel P, Corash L, Koutsoukos A, Lin L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood. 104: 1534-41, 2004.
Mykhailova O, Turner TR, Olafson C, Howell A, Nahirniak SN, Wizniak J, Gerges HYN, Baldwin T, Clarke G, Acker JP. Hypothermic storage of leukoreduced red blood cells for greater than 21 days is a safe alternative to irradiation. Transfusion. 2021 Apr;61(4):1247-1257.
National Advisory Committee on Blood and Blood Products. Terms of Reference [Internet]. Canada; National Advisory Committee on Blood and Blood Products; 2021 Jun 10. [cited 2022 Oct 30]. Available from: https://nacblood.ca/en
National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. Division for Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. Atlanta, GA, USA. 2023 Feb. [cited 2023 Apr 17]. Available from: https://www.cdc.gov/nhsn/pdfs/biovigilance/bv-hv-protocol-current.pdf
Pineda A, McCullough J, Benjamin RJ, Cable R, Strauss RG, Burgstaler E, Porter S, Lin L, Metzel P, Conlan MG; SPRINT Study Group. Pathogen inactivation of platelets with a photochemical treatment with amotosalen HCl and ultraviolet light: process used in the SPRINT trial. Transfusion. 46: 562-71, 2006.
Rühl H, Bein G and Sachs UJH. Transfusion-associated graft-versus-host disease. Transfusion Medicine Reviews 23: 62-71, 2009.
Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. Journal of Medical Genetics 34: 798-804, 1997.
Serrano K, Chen D, Hansen AL, Turner TR, Kurach JDR, Acker JP and Devine DV. The effect of timing of gamma-irradiation on hemolysis and potassium release in leukoreduced red cell concentrates stored in SAGM. Vox Sanguinis 106: 379-381, 2014.
Serrano K, Pambrun C, Levin E, Devine DV. Supernatant reduction of stored gamma-irradiated red blood cells minimizes potentially harmful substances present in transfusion aliquots for neonates. Transfusion 57: 3009-18, 2017.
Spitzer TR, Cahill R, Cottler-Fox M, Treat J, Sacher R, Deeg HJ. Transfusion-induced graft-versus-host disease inpatients with malignant lymphoma. A case report and review of the literature. Cancer 66: 2346-9, 1990.
Snyder E, McCullough J, Slichter SJ, Strauss RG, Lopez-Plaza I, Lin JS, Corash L, Conlan MG; SPRINT Study Group. Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation: the SPRINT trial. Transfusion. 45: 1864-75, 2005.
Trudeau JD, Waters T and Chipperfield K. Should intraoperative cell-salvaged blood be used in patients with suspected or known malignancy? Canadian Journal of Anesthesia. 59: 1058-1070, 2012.
Uchida S, Tadokoro K, Takahashi M, Yahagi H, Satake M and Juji. Analysis of 66 patients with definitive transfusion-associated graft-versus-host disease and the effect of universal irradiation of blood. Transfusion Medicine, 23: 416-422, 2013.
United Nations Children’s Fund and World Health Organization, Low Birthweight: Country, regional and global estimates. UNICEF, New York, 2004. http://apps.who.int/iris/bitstream/10665/43184/1/9280638327.pdf
van Rhenen D, Gulliksson H, Cazenave JP, Pamphilon D, Ljungman P, Klüter H, Vermeij H, Kappers-Klunne M, de Greef G, Laforet M, Lioure B, Davis K, Marblie S, Mayaudon V, Flament J, Conlan M, Lin L, Metzel P, Buchholz D, Corash L; euroSPRITE trial. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood. 101: 2426-33, 2003.
van Rhenen DJ, Gulliksson H, Cazenave JP, Pamphilon D, Davis K, Flament J, Corash L. Therapeutic efficacy of pooled buffy-coat platelet components prepared and stored with a platelet additive solution. Transfusion Medicine. 14: 289-95, 2004.
Waters JH, Yazer M, Chen Y, and Kloke J. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 10: 2167-73, 2012.
Wiersum-Osselton JC, Slomp J, Falkenburg JHF, Geltink T, van Duijnhoven HLP, Netelenbos T, Schipperus MR. Guideline development for prevention of transfusion-associated graft-versus-host disease: reduction of indications for irradiated blood components after prestorage leukodepletion of blood components. British Journal of Haematology. 195: 681-8, 2021.
Williamson LM, Stainsby D, Jones H, et al. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft versus-host disease. Transfusion 47: 1455-1467, 2007.
Zimmermann R, Schoetz AM, Frisch A, Hauck B, Weiss D, Strobel J and Echstein EtR. Influence of late irradiation on the in vitro RBC storage variables of leucoreduced RBCs in SAGM additive solution. Vox Sanguinis 100: 279-284, 2011.
Appendices
Quick reference of clinical indications for irradiated blood component transfusion.