Recommendations for the Notification of Recipients of a Blood Component Recall
Tanya Petraszko, MD (Co-Chair)
Ann Tran, MD
Lakshmi Rajappannair, MD
Robert Liwski, MD
Marissa Laureano, MD
Mark Bigham, MD
Mindy Goldman, MD
Steven Drews, PhD
List of abbreviations
Creutzfeldt-Jakob Disease
Cytomegalovirus
Monkey Pox
Transfusion-Transmission of Malaria
Variant Creutzfeldt-Jakob Disease
Canadian Blood Services
Epstein Barr Virus
National Advisory Committee on Blood and Blood Products
National Recipient Advisory Committee
Not Applicable
Nucleic Acid Testing
Provinces and Territories
Provincial / Territorial Canadian Blood Services Blood Liaison Committee
Transfusion Related Acute Lung Injury
Summary of Revisions
Revision Date
Details
Associated Blood Component – A blood component that is directly associated in a recall (i.e. packed red blood cell unit).
Checkmark – Indicates affirmation for that column. Please see antithetical column for any exceptions.
Companion Blood Component – A blood component that has been produced from the same donation (i.e. pooled platelet unit from the same donation as the implicated red blood cell unit).
Large Scale Recall – A recall of 50 or more blood components OR a recall of a small number of blood components involving multiple provinces. This does not include reasons for common recalls outlined in this document.
NA – Indicates “Not Applicable” for that column. Please see notes in the antithetical column.
Notification Not Recommended – If this applies, then further investigation/action is not required.
Notification Recommended – If this applies, then an investigation is recommended which may include patient chart review, discussion with the patient’s clinician for additional information or testing recommendations, and/or patient notification.
Recall – The removal from further distribution, or use, of a product (blood component) that violates legislation administered by Health Canada (a regulatory requirement).
Unusual Recall – A recall due to an unanticipated event impacting a large or small number of blood components.
Withdrawal – The voluntary removal by the manufacturer (blood supplier) of a product (blood component) that does not violate legislation administered by Health Canada.
The National Advisory Committee on Blood and Blood Products (NAC) is an interprovincial medical and technical advisory body to the Provincial and Territorial (P/T) Health Ministries and the blood supplier Canadian Blood Services (CBS). Its mandate is to provide professional leadership and advice in matters directly affecting the practice of transfusion medicine in hospitals. In 2010, NAC was asked by the Provincial/Territorial Canadian Blood Services Blood Liaison Committee (P/T CBS BLC) to:
- Develop national recommendations ensuring consistent recipient notification when a recall or a withdrawal of a blood component has occurred;
- Identify who is responsible for each stage of the notification process; and,
- Recommend a group of resource experts that would be convened in the event of an unusual or large scale recall/withdrawal to provide direction regarding recipient notification for a situation that is not specifically addressed in the national recommendation document.
NAC collaborated with CBS in the development of this recommendation document. It is recommended that this document be used as a reference by hospital transfusion services, the blood supplier, and P/T representatives.
Although the definitions for a recall and a withdrawal of blood components differ (refer to the list of definitions), from a practical perspective the consequences for the blood component is the same. The blood supplier removes the blood component from inventory and hospital transfusion services supplied by CBS are notified if they have received associated or companion blood components. This document does not distinguish between the two terms. The term ‘recall’ is used as a comprehensive term throughout the remainder of the document.
The recommendations for recipient notification outlined in this document are the suggested actions required in terms of notification. The general recommendations presented are applicable to causes of blood component recalls that are known to occur and initiated by the blood supplier; the blood supplier being CBS in all P/Ts with the exception of Quebec. This document does not prevent P/Ts or individual hospitals from implementing notification processes above and beyond what has been recommended, or modifying processes as determined locally.
It is recommended that all hospitals have their own policies and procedures for the process of notification in accordance with applicable P/T regulations. Throughout this document when notification is recommended every hospital should have an internal procedure outlining who is responsible for notification and the process by which it should occur. It is recommended that local risk management be consulted in the development of this process.
This document does not address recalls initiated due to a donor testing positive for a transmissible disease test such as Hepatitis B, Hepatitis C, HIV, HTLV, Syphilis, WNV or T.cruzi (Chagas disease). In these instances, CBS indicates to the hospital the required actions via standard lookback procedures.
This document currently only addresses recipient notification in terms of recalls associated with the transfusion of fresh blood components collected, produced and/or distributed by CBS (i.e., red blood cell, platelet and frozen plasma components). However, there may be applicability to a recall of fractionated or recombinant plasma protein products (i.e. an unusual or large scale recall) that may result in the National Recipient Advisory Committee (NRAC) being convened with is discussed further in Section 7.
These recommendations for notification have been made in consideration of the available literature. There are clinical situations that the treating physician should consider when further evaluating the recommendations for notification contained in this document. These conditions include, but are not limited to:
- Presence or absence of symptoms during or after transfusion (relevant for possible bacterial contamination, malaria risk);
- Pregnancy (relevant for teratogenic drugs);
- Underlying condition (relevant if patient is immunocompromised and donor developed EBV infection);
- The age of the patient; and,
- Relevant prognosis.
Depending on the age and prognosis of the recipient, the treating physician may consider notifying next of kin or family members as an alternative to notifying the recipient directly about a blood component recall. This should be done in accordance with applicable provincial regulations.
For instances where recipient notification is not recommended and a review of recipient records are required to confirm this, it is recommended that hospitals maintain a record of their activities related to the review of information as appropriate. Recipient notification and any follow‐up testing should occur as soon as possible in relation to the relative risk that is associated with the cause of the blood component recall.
It is recommended that consultation with a CBS Medical Officer occur as necessary should further information or clarification be required with respect to any notification received regarding a blood component recall.
Recalls and withdrawals of blood products are initiated by CBS in accordance with Health Canada regulations and standard operating procedures.
Once CBS makes the decision to recall or withdraw blood components from inventory, the required notification to hospitals is conducted as per standard procedure. If upon notification the hospital concludes that the identified blood component was transfused, a decision with regard to recipient notification must be made.
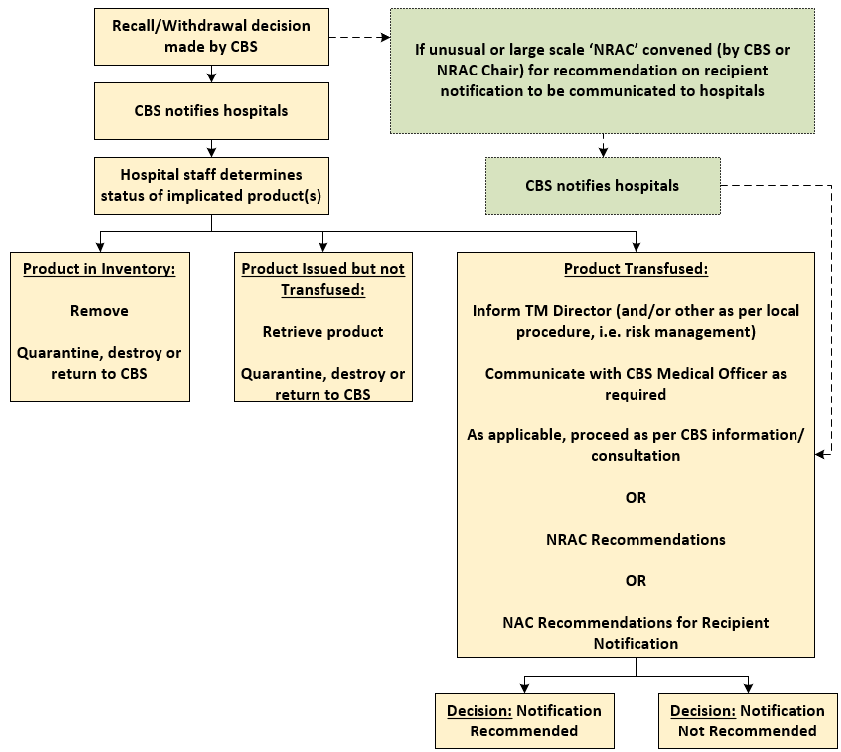
For recalls associated with infectious disease markers, CBS will provide direction to hospitals regarding recipient notification in accordance with lookback procedures. As per Figure 1, found below, if the reason to recall a blood component is unusual or if the recall involves a large amount of blood components, a recommendation for recipient notification may be made by the NRAC. The recommendation made by the NRAC will be communicated to hospitals by CBS through regular communication methods. In these instances, hospital reference to the NAC Recommendations for Recipient Notification is not required unless information with respect to the functionality and scope of the NRAC is needed.
Figure 1: Flow chart indicating the process prior to recipient notification being conducted.
The following sections (3.0 to 6.0) provide recommendations for notification depending on the reason for recall. The following definitions apply to all 4 sections:<
Notification Recommended – If this applies, then an investigation is recommended which may include patient chart review, discussion with the patient’s clinician for additional information or testing recommendations, and/or patient notification.
Notification Not Recommended – If this applies, then further investigation/action is not required.
NA – Indicates “Not Applicable” for that column. Please see notes in the antithetical column.
Checkmark – Indicates affirmation for that column. Please see antithetical column for any exceptions
Table 1: Recalls initiated as a result of post donation information received by the blood supplier.
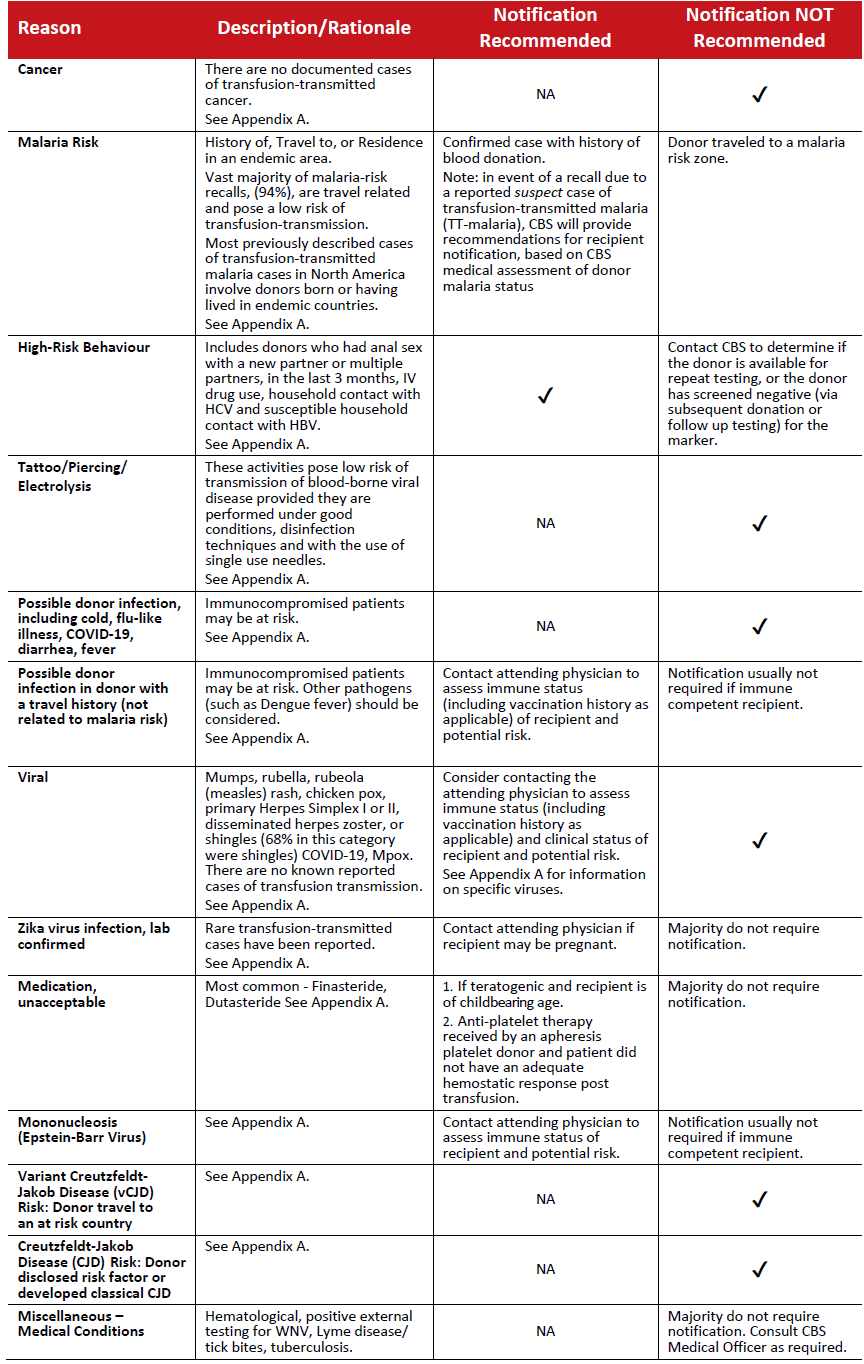
Note: The reasons listed above account for over 90% of the total annual recalls in this category (based on CBS 2021/2022 data).
Table 2: Recalls initiated by the blood supplier owing to technical manufacturing issues.
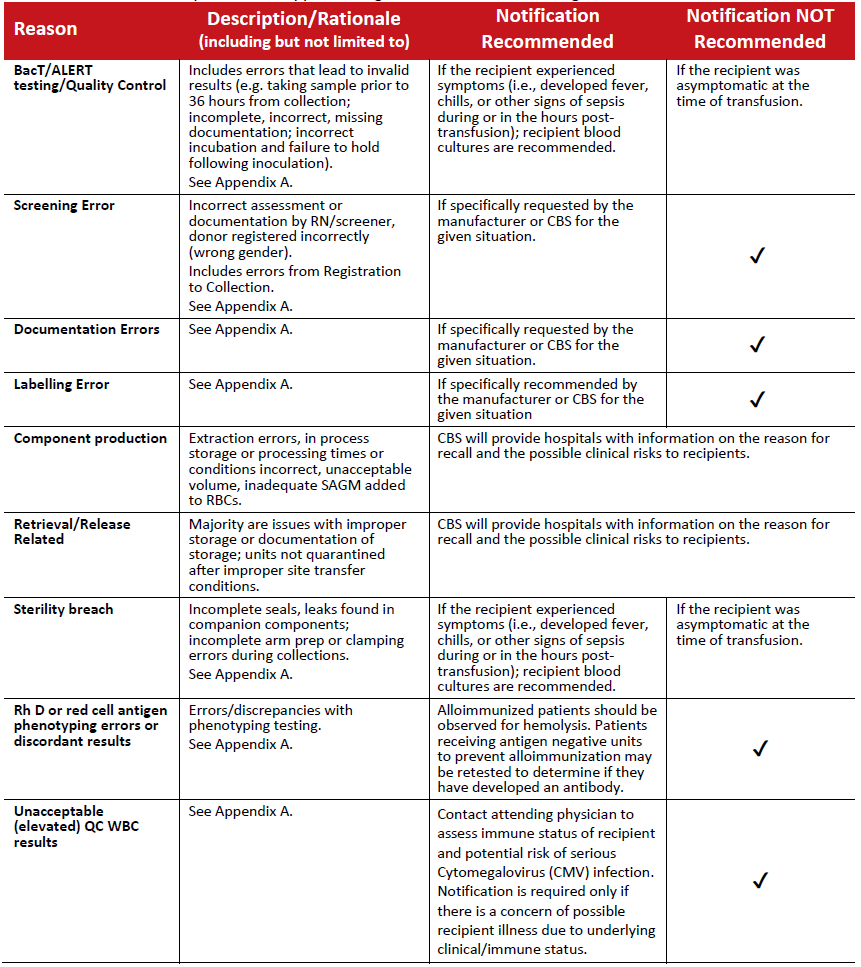
Note: The reasons listed above account for over 90% of the total annual recalls in this category (based on CBS 2021/2022 data).
Table 3: Recalls initiated as a result of the possibility of bacterial contamination in a blood component.
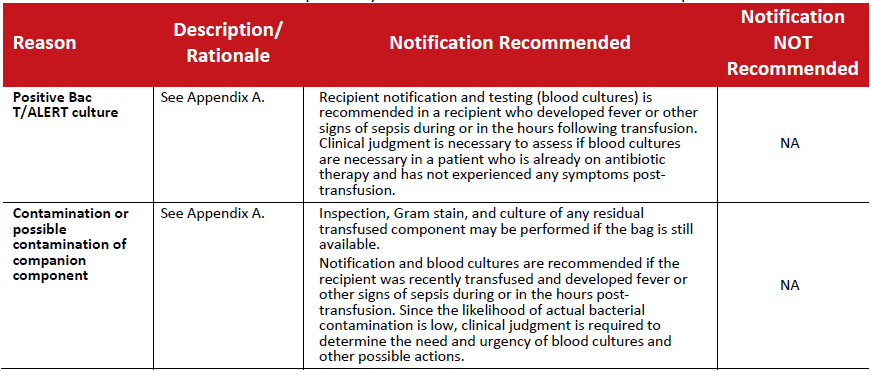
Table 4: Recalls initiated as a result of a reported transfusion reaction ‐ TRALI.
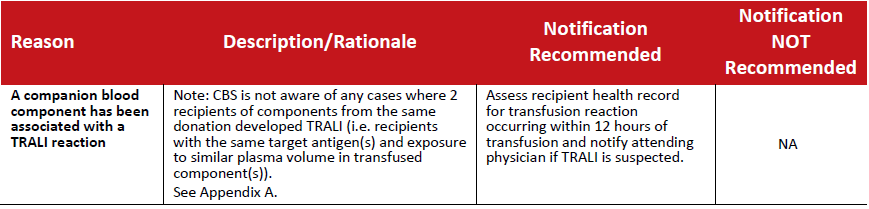
In the event that an unusual situation triggers a recall of blood components, or a large number of blood components are involved in a recall, it is recommended that the National Recipient Advisory Committee (NRAC) be convened to make recommendations regarding recipient notification. This group of resource experts may also be convened to provide recommendations regarding recipient notification for recall situations that are not currently addressed in the preceding sections of this document.
CBS will accept the NRAC recommendation as the principal consideration in rendering a decision regarding recipient notification.
The Terms of Reference for this committee are as follows:
7.1 Mandate
The NRAC will develop recommendations and provide advice to CBS with respect to recipient notification in the event of a blood component recall situation that involves a large number of blood components or if the situation is not currently addressed in available national recommendations.
To this end the NRAC will:
- Discuss if recipient notification is required;
- Develop a recommendation for consistent recipient notification across the country with respect to specific recall scenarios;
- Indicate if follow‐up testing should be considered for recipients that were transfused impacted blood components; and,
- Provide recommendations regarding the communications issued to hospitals via CBS.
7.2 Membership
The Chair of the NRAC will be the current chair of the National Advisory Committee on Blood and Blood Products (NAC). The Vice Chair of NAC shall act as chair in the absence of the NAC Chair.
Membership of the NRAC includes the following:
- CBS Chief Supply Chain Officer;
- NAC Chair and Vice Chair (or designates);
- CBS Vice President, Medical Affairs and Innovation;
- Blood Portfolio Lead Province Representative; and,
- Depending on the size and nature of the recall, further experts may be engaged on an ad hoc basis including but not limited to:
- NEBMC members;
- Ethicist;
- A blood transfusion recipient representative; either an actual recipient or a ‘representative’ of an appropriate patient society; and,
- Public Health Agency of Canada representative.
7.3 Meetings
Meetings will be convened at the request of CBS or the Chair as required. Decisions of the NRAC will be made by consensus. Consensus is defined as 80% (or greater) agreement of the voting members present.
In the event that consensus cannot be reached, CBS will make decisions considering the advice received from the NRAC.
The National Emergency Blood Management Committee Secretariat will arrange teleconferences/meetings, record and distribute meeting minutes and the record of decisions and maintain the membership list and respective contact information for the NRAC.
NAC and CBS wish to acknowledge Dr. Mindy Goldman and Dr. Margaret Fearon, Canadian Blood Services and Nancy Heddle, McMaster University, for their valued contributions in the development of the initial version.
References
GENERAL
Canadian Disclosure Guidelines. Canadian Patient Safety Institute; May 2011. Available online at: https://www.patientsafetyinstitute.ca
Heddle, Nancy M. et al. "TRANSFUSION SERVICE: A policy informing qualitative study to improve the process of blood product recalls and withdrawals." Transfusion 48.12 (2008):2585‐ 2595.
Recommendations pour la Notification des Receveurs a la Suite d’un Retrait de Produits Sanguins. Quebec : Gouvernement du Quebec. SeSS; 2004.
Eder AF, Goldman M. Post-donation Information and Blood Component Retrievals: Realigning Blood Center and Hospital Actions Based on Risk Assessment. Transfus Med Rev 2014; 28:226‐ 234.
Ramsey G. Blood component recalls and market withdrawals: frequency, reasons, and management in the United Status. Transfus Med Rev 2013; 27:82‐90.
Lindholm PF, Annen K, Ramsey G. Approaches to minimize infection risk in blood banking and transfusion practice. Infect Disord Drug Targets 2011; 11(1):45‐56.
BacT/ALERT, INVALID TESTING
Ramirez-Arcos S, Evans S, McIntyre T, Pang C, Yi Q-L, DiFranco C, Goldman M. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-2928.
BACTERIAL CONTAMINATION
Positive BacT/ALERT, contamination or possible contamination of companion component Bacterial Contamination of Platelets: Summary for Clinicians on Potential Management Issues Related to Transfusion Recipients and Blood Donors. AABB Bacterial Contamination Task Force, Feb 23, 2005. Available online at: https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-blood/platelet-components.
Guideline for Investigation of Suspected Transfusion Transmitted Bacterial Contamination, PHAC, CCDR 2008;34S1. Available online at: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2008-34/guideline-investigation-suspected-transfusion-transmitted-bacterial-contamination.html.
Eder A, Goldman M. How do I investigate septic transfusion reactions and blood donors with culture‐positive platelet donations. Transfusion 2011; 51:1662.
CANCER
Edgren G, Hjalgrim H, Reilly M, et al. Risk of cancer after blood transfusion from donors with subclinical cancer: a retrospective cohort study. The Lancet 2007; 369:1724‐1730.
Yang H, Lee J, Seed CR, Keller AJ. Can Blood Transfusion Transmit Cancer? A Literature Review. Transfus Med Rev 2010; 24:235‐243.
Hjalgrim H, Rostgaard K, Vasan SK, et al. No evidence of transmission of chronic lymphocyte leukemia through blood transmission. Blood 2015;126:2059-2061.
CJD
Urwin PJ, Mackenzie JM, Llewelyn CA, Will RG, Hewitt PE. Creutzfeldt‐Jakob disease and blood transfusion: updated results of the UK Transfusion Medicine Epidemiology Review study. Vox Sang. 2016;110:310‐316.
Crowder LA, Schonberger LB, Dodd RY, Steele WR. Creutzfeldt-Jakob disease lookback study: 21 years of surveillance for transfusion transmission risk. Transfusion 2017;57:1875-1878.
vCJD RISK AREAS (travel or transfusion)
FDA Guidance for Industry: Recommendations to Reduce the Possible Risk of Transmission of Creutzfeldt‐Jakob Disease and Variant Creutzfeldt‐Jakob Disease by Blood and Blood Components, May 2022. Available online at:
Seed CR, Hewitt PE, Dodd RY, Houston F, Cervenakova L. Creutzfeldt‐Jakob disease and blood transfusion safety. Vox Sang. 2018;113:220‐231.
McManus H, Seed CR, Hoad V, et al. Risk of variant Creutzfeldt-Jakob disease transmission by blood transfusion in Australia. Vox Sang 2022;117:1016-1026.
University of Edinburgh CJD International Surveillance Network. At: https://www.eurocjd.ed.ac.uk/data_tables. Accessed 13 Jan 2023
COVID-19 (SARS-CoV-2)
Leblanc JF et al. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: A literature review. Transfusion 2020;60:3046-3054.
Kiely P et al. Severe Acute Respiratory Syndrome Coronavirus 2 and blood safety: an updated review. Transfus Med Hemother 2022;49;218-228.
Cappy P, et al. SARS-CoV-2 and post-donation information: a one-year experience of the French haemovigilance network. Blood Transfusion 2022;20:362-373.
Luzzi JR, et al. COVID-19: Further evidence of no transfusion transmission. Transfusion and Apheresis Science 2021;60:102961.
HIGH RISK BEHAVIOUR
O’Brien SF, Yi QL, Fan W, et al. Residual risk of HIV, HCV and HBV Canadian. Transfusion and Apheresis Science 2017;56:389-391.
Canadian Blood Services Annual Surveillance Report, 2021. Available online at: https://professionaleducation.blood.ca/en/transfusion/publications/surveillance-report.
INFECTION
Possible donor infection, including cold, flu, diarrhea, fever
Goldman M, Long A, Roy G, et al. Incidence of Positive Bacterial Cultures after Donor Call‐Back. Transfusion 1996; 36:1035.
Eder AF, Goldman M. How do I investigate septic transfusion reactions and blood donors with culture‐positive platelet donations? Transfusion 2011; 51:1662‐1668.
Possible Viral Infection
AABB website: https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/infectious-diseases/emerging-infectious-disease-agents/fact-sheets-created-or-updated-post-publication-of-the-transfusion-august-2009-supplement. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion 2009 Supplement 49.
Includes fact sheets for:
- Herpes simplex viruses 1 and 2, varicella zoster (chicken pox, shingles)
- Influenza A or B viruses
- Mumps virus
- Mononucleosis (Epstein‐Barr Virus)
- Zika virus
- and many other pathogens
MALARIA
Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion‐transmitted malaria in the United States from 1963 through 1999. N Engl J Med 2001; 344:1973‐1978.
Mace KE, Lucchi NW, Tan KR. Malaria Surveillance – United States, 2017. MMWR 2021. Available online at: https://www.cdc.gov/mmwr/volumes/70/ss/ss7002a1.htm.
Spencer B, Steele W, Custer B, Kleinman S, et al. Risk for malaria in United States donors deferred for travel to malaria‐endemic areas. Transfusion 2009; 49:2335‐2345.
Spencer B, Kleinman S, Custer B, et al. Deconstructing the risk for malaria in United States donors deferred for travel to Mexico. Transfusion 2011; 51(11):2398‐2410.
MEASLES, MUMPS, RUBELLA
Forthal DN, Aarnaes S, Blanding J, de la Maza L, Tiles JG. Degree and length of viremia in adults with measles. Journal of Infectious Diseases. 1992;166:421‐4
Shin S, Lee S, Cho Y, Shin Y. Blood‐borne transmission of the measles, mumps, and rubella vaccine virus. Transfusion 2011;51:663‐664
MEDICATION, UNACCEPTABLE
Han JY, Choi JS, Chun JM, Park HD, et al. Pregnancy outcome of women transfusion during pregnancy with blood products inadvertently obtained from donors treated with acitretin. J Obs Gyn 2009; 29:694‐697.
Geiger JM, BoudinM, Saurat JH. Teratogenic Risk with etretinate and acitretin treatment. Dermatology 1994;189:109-116.
MPOX (MONKEYPOX)
Monkey pox Virus – Interim Fact Sheet. Available online at:
Dodd RY, Stramer SL. Monkeypox and transfusion safety. Transfusion Medicine Reviews 2022:14:49
TATTOO / PIERCING / ELECTROLYSIS
Goldman M, Xi G, Yi QL, Fan W, et al. Reassessment of deferrals for tattooing and piercing. Transfusion 2009; 49:648‐654.
Hoad V, Guy RJ, Seed CR, Harley R. Tattoos, blood-borne viruses and blood donors: a blood donor cohort and risk assessment. Vox Sang 2019;114:687-693.
Prinsze FJ, van de Laar T, Slot E, et al. No increased risk of transfusion-transmissible infections after tattooing, body piercing, or acupuncture among blood donors in the Netherlands. Transfusion 2019;59:2575-2583.
TRALI
Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 2019;133:1840-1853.
VARICELLA, ZOSTER, HERPES SIMPLEX I & II
Hudnall SD, Chen T, Allison P, Tyring SK, Heath A. Herpesvirus prevalence and viral load in healthy blood donors by quantitative real time polymerase chain reaction. Transfusion 2008;48:1180‐1187.
Kimura H, Kido S, Ozaki T, et al. Comparison of quantitation’s of viral load in Varicella and Zoster. Journal of Clinical Microbiology. 2000; 38(6) :247.
Chan HM, Ho PL, Chan KH, Lin CK, Lee CK. Interdiction of a blood donation containing varicella‐zoster virus by donor self‐report of chickenpox. Vox Sang 2013; 104:248‐249.
ZIKA VIRUS
Barjas‐Castro ML, Angerami RN, Cunha MS et al. Probable transfusion‐transmitted Zika virus in Brazil. Transfusion. 2016;56:1684‐1688.
Saá P, Proctor BS, Foster BA et al. Investigational testing for Zika virus among U.S. blood donors. NEJM. 2018;378:1778‐88.
Appendices
POST-DONATION INFORMATION
Cancer
Donors who have had most forms of cancer are temporarily deferred from donation until recurrence-free for a specified time period. Donors reporting a diagnosis of recent cancer post‐donation are a frequent cause of post‐donation information. In-date components at the time the information is obtained (up to 12 months from donation for plasma or cryoprecipitate) are recalled.
A large Scandinavian cohort study of cancer incidence in recipients of blood from donors who had subclinical cancer at the time of donation demonstrated no excess risk of cancer compared to recipients who had received blood from donors who did not develop cancer post‐donation. The FDA found no evidence that development of cancer in donors affected the safety, purity, or potency of blood components, and does not require recall of components after a diagnosis of cancer is made in donors. There is no evidence of transfusion-transmission of solid-organ cancer.
Malaria Risk
Recalls are usually due to unreported malaria risk travel. Cellular components are recalled if the donor left an at risk area 6 months or less prior to donation. The overall risk of transfusion‐ transmission of malaria (TT-malaria) in the US and Canada is less than 1 per million cellular components transfused. From 1997 to 2021, there were no cases of TT-malaria reported in Canada, and approximately 1 to 2 cases of TT-malaria reported annually in the US. A probable TT-malaria case was described in Canada in 2022. Cases are most commonly associated with donors who are immigrants from malaria‐risk areas. Short‐term travel to malaria-endemic areas poses a very low risk of TT-malaria. In addition, a 21 day post-travel donor deferral for travel outside Canada, continental US, or Europe further reduces risk of travel-associated infections like malaria and other arbovirus infections such as Dengue, Chikungunya or Zika. Symptoms and signs of post‐transfusion malaria include fever, fatigue, anemia, and altered mental status, usually developing from 2 to 3 weeks post‐transfusion for falciparum malaria. The incubation period may be longer after other species, and up to 73 days for P. malariae.
High Risk Behaviour
Occasionally, donors report high risk behaviour, such as illicit intravenous drug use that would have led to indefinite deferral. Components are recalled if such behaviour occurred recently. Since infectious disease testing at the time of donation was negative, risk of disease transmission is likely extremely low. At the present time, CBS analytic detection window periods for HIV, HCV, and HBV are estimated at 6.1, 2.9, and 18.8 days, respectively. However, recipient infection with any of these viruses may be asymptomatic at the time of transfusion but have major health consequences both for the individual and their household and sexual contacts.
Possible donor infection, including cold, flu-like illness, COVID-19, diarrhea, fever
Donors who develop symptoms such as fever, chills, and diarrhea in the days post‐donation may have been bacteremic at the time of donation. Culture of all platelet components has provided more information about possible sources of asymptomatic bacteremia in donors. Donors in the incubation phase of a gastrointestinal or upper respiratory tract bacterial infection are extremely rarely associated with positive bacterial cultures. Generally, common, often seasonal, community-acquired respiratory viruses are not considered transfusion-transmissible.
Possible donor infection in donor with a travel history (not related to malaria risk)
Donors with a travel history who develop symptoms such as fever, joint pain with or without rash and conjunctivitis necessarily have a broader differential diagnosis than donors who have not traveled abroad. Discussion with the CBS Medical Officer is recommended, as further information/testing may narrow the etiology, as is correlation with clinical signs/symptoms in the recipient. For donors with a travel history to areas of malaria risk, refer to Malaria Risk above.
Tattoo, piercing, electrolysis
A precautionary 3 month temporary donor deferral is applied after tattooing, any piercing, and after electrolysis with non‐single use needles. Donors who return after temporary deferral have not been found to have an increased risk of positive HBV or HCV infectious markers compared to other donors. There appears to be little increased risk associated with these activities occurring in these time frames prior to donation.
Medication, unacceptable
Blood is recalled if donors are identified to be taking highly teratogenic medications that have been associated with birth defects when taken by pregnant women. However, there is limited data on the risk of a one‐time exposure through blood transfusion. The one published study did not show any adverse outcomes such as malformations in infants of women taking one of these medications, acitretin, before or during pregnancy. Therapeutic efficacy of platelets may be decreased if a donor was taking medication with anti‐platelet effects, such as ASA, at the time of donation. Depending on the medication, some of the platelet function defect may be reversible after transfusion.
Travel or transfusion, vCJD risk area
There have been four probable cases of Variant Creutzfeldt-Jakob Disease (vCJD) transmission through blood transfusion. All these cases occurred in the UK. The involved donors developed vCJD from 17 months to 3.5 years after donation. Since 2019, 232 vCJD cases have been reported in 12 countries, the majority in the UK (178) and France (28). Non‐UK cases have been linked to previous residence in the UK or residence in other countries with a risk of Bovine Spongiform Encephalopathy in the food chain from meat imported from the UK. The risk of transmission of vCJD from donors who have resided in or have been transfused in a vCJD risk area but have not developed vCJD is therefore extremely small.
Health Canada directives do not contain any information regarding notification of recipients. However, the FDA Guidance document referenced above specifically mentions that the FDA does not believe it is appropriate to conduct tracing and notification of recipients of components from donors who have resided in or been transfused in vCJD geographic risk areas.
Classical CJD risk
Donors are deferred for use of human pituitary derived growth factor and gonadotrophin hormones, and for a history of Creutzfeldt-Jakob Disease (CJD) in family members. Donors may report that they or a family member have developed CJD or did not report risk at the time of donation.
Two large cohort studies, one in the UK and one in the US, followed recipients who received blood components from donors who developed CJD. To date, after more than a decade of follow up in over 100 recipients, there has been no evidence of CJD transmission. The FDA Guidance document referenced above specifically mentions that the FDA does not believe it is appropriate to notify recipients of components from donors who develop CJD themselves or whose family members develop CJD.
Mononucleosis (Epstein‐Barr Virus)
As a precautionary measure, all components donated in the 30 days prior to development of mononucleosis are recalled. Transmission of EBV has been documented in immunosuppressed recipients, especially following transplantation. Since EBV is a B lymphocyte associated virus, risk of transmission is substantially decreased by universal leukoreduction. Additionally, life-time chronic carriage of the virus occurs in over 90% of the adult population. Therefore, most recipients would be expected to be already infected.
Mpox
Monkey pox (Mpox) disease emerged from Africa in winter-spring of 2022. There have been no reported cases of transfusion-transmitted Mpox, although transmission by this route is considered theoretically possible. CBS added eligibility questions about Mpox in Aug 2022. Mpox infection requires a 42 day deferral from symptom onset. Close contact with a Mpox case in the previous 6 weeks requires a 42 day deferral from last contact. Close contact is defined as contact with skin, body fluids, or a contaminated object such as clothing, as well as face-to-fact contact without a mask for more than three hours. A medical review will be undertaken for each case in a donor in the post-donation period.
COVID-19 (SARS-CoV-2)
In spite of millions of COVID-19 cases world-wide, transfusion-transmission of the SARS-CoV-2 virus has not been demonstrated, and several studies suggest that transfusion-transmission does not occur or is so exceedingly rare that it has not been detected. By the fall of 2022 over 95% of CBS donors were vaccinated, and over 60% have already had a SARS-CoV-2 natural infection. In the instance that CBS receives post-donation information from donors about the development of recent COVID-19 post-donation (i.e. symptom onset within 10 days of donation), components will be withdrawn from inventory. No additional recipient follow up is recommended.
Viral
As a precautionary measure, all components are recalled when donors are diagnosed with symptoms of these infections developing up to 7 days post‐donation. Theoretically, the donor may have been viremic at the time of donation. In practice, transmission of these agents has rarely, if ever, been documented.
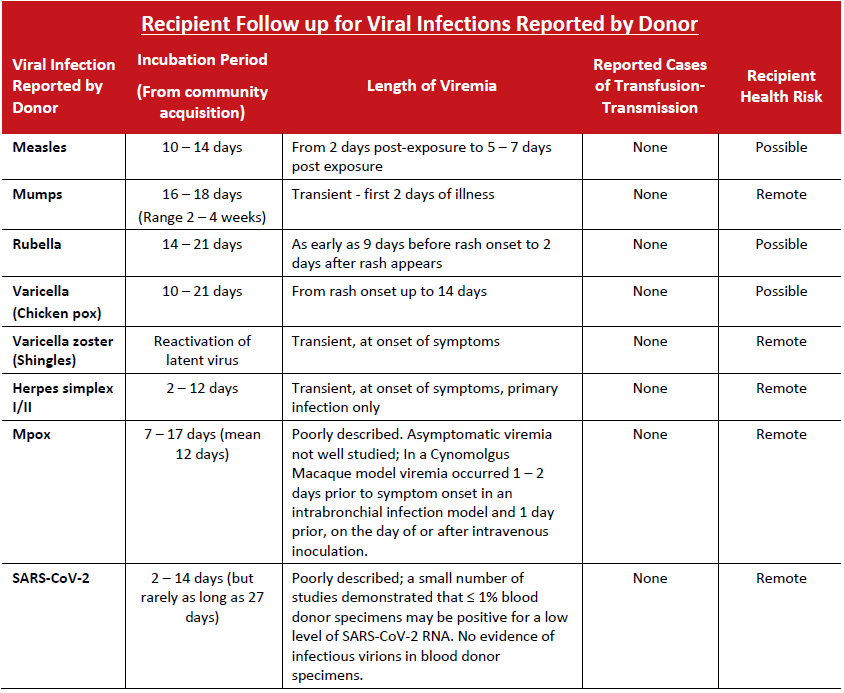
Zika virus infection, lab confirmed
Probable cases of transfusion‐transmitted Zika virus infection have been rarely reported, and in these instances recipients were asymptomatic. Correlation with the patient’s pregnancy status is important, as Zika virus is unlikely to be a significant risk to non‐pregnant individuals. For pregnant recipients, follow up including testing for Zika virus is recommended.
TECHNICAL MANUFACTURING ISSUES
Invalid BacT/Alert Testing
Testing may not have been properly performed (for example, sample taken less than 36 hours post collection), or machine malfunction may have invalidated the results of BacT/ALERT culture. Since the platelet component did not have adequate testing performed, there is an increased risk of bacterial contamination of approximately 1 in 10,000 (rate of true positive bacterial cultures of platelets at CBS).
Documentation errors ‐ donor screening and production
Documentation errors on the CBS Record of Donation or during component manufacturing are most often due to incomplete information, such as lack of a signature or missing documentation of storage time. These are errors of Good Manufacturing Process (GMP) therefore the product is recalled. However, it is extremely unlikely that there is any additional risk associated with transfusion of the component.
Labeling Errors
Errors may occur in the product code listed on the label, the volume of the component, or any other product attribute listed on the label. These are errors of GMP, therefore the product is recalled. However, in most cases, there is no additional risk associated with transfusion of the component. Exceptionally, there may be recipient risk; an example would be an incorrect ABO group.
Sterility Breach
Incidents such as incomplete seals may lead to a slightly increased risk of bacterial contamination of the component. The risk will vary depending on the component involved and the exact problem that occurred.
Rh D or red cell antigen phenotyping errors or discrepancies
These errors may be of importance if the recipient is alloimmunized against the mistyped antigen (for example, unit now known to be Kell positive transfused to recipient with anti‐K); a delayed hemolytic transfusion reaction may occur. A recipient receiving units erroneously typed as antigen negative to prevent alloimmunization may actually develop an antibody. Discrepancies may be related to weak subgroups of A or B, or weak or partial D antigens.
Unacceptable Quality Control, WBC Counts
Products with elevated white blood cell counts over the maximum permitted in the CBS Circular of Information may have an increased risk of transmission of Cytomegalovirus (CMV). Transmission of CMV may be of major clinical significance in CMV seronegative recipients receiving a CMV seropositive product. Patients at risk for significant transfusion‐transmitted CMV disease include recipients of allogeneic stem cell transplants from CMV seronegative donors.
BACTERIAL CONTAMINATION
Positive BacT/ALERT culture
Donation components will be recalled if the BacT/ALERT automated culture system indicates a positive reaction. Further information may follow the initial report. Subsequent investigation may show actual bacterial contamination (true positive), as well as identification of the organism. In other cases, further investigation may indicate a false positive reaction. Bacterial proliferation is most common in platelet units, however septic reactions have been reported with red cell and frozen components as well. Signs and symptoms of post‐transfusion sepsis include fever, chills, and hypotension usually developing during or in the four hours post‐transfusion. Investigation of a suspected reaction may include inspection, Gram stain, and culture of any residual component, if available. In addition, patient blood cultures may be indicated, particularly if recall notification is obtained shortly after transfusion, or the recipient has developed fever and chills post‐transfusion.
Contamination or possible contamination of companion component
All components of a donation are recalled if a positive BacT/ALERT culture is found on an individual component, or if CBS is informed of a possible septic reaction in a patient having received a transfusion with one component from the donation. Further clarifying information may follow, such as repeat culture results or organism identification. In the case of positive bacterial cultures on a buffy coat platelet pool, all red cell and plasma components associated with the pool will be recalled. A small minority of these companion products will actually be bacterially contaminated.
TRANSFUSION RELATED ACUTE LUNG INJURY
TRALI, increased risk
Components may be recalled for increased Transfusion Related Acute Lung Injury (TRALI) risk if a companion blood component from the same donation or a component from a later donation from the same donor has been associated with a TRALI reaction.
The pathogenesis of TRALI is poorly understood. The presence of an anti‐HLA or anti‐granulocyte antibody in the donor directed against the cognate antigen in the recipient, plasma content of the component, and underlying disease in the recipient all play a role in the development of a TRALI reaction. In lookback studies performed on recipients of components from donors who have been clearly implicated in an antibody mediated TRALI reaction, the frequency of pulmonary complications and TRALI is slightly higher than in transfusion recipients in general. However, the majority of recipients did not develop TRALI reactions.
