Overview of Adverse Transfusion Reaction Reporting for Hospitals in Canada: a NAC and Québec Hemovigilance System Collaborative Initiative
Alan Tinmouth, MD
Lakshmi Rajappannair, MD
Lucinda Whitman, MD
Ad hoc members:
Mohammad Afzal (PHAC)
Maria Faraci, MD (Health Canada)
Gilles Lambert, MD (QHS/INSPQ)
Marianne Lavoie, MD (QHS/INSPQ)
Jessica Leung, PharmD (Health Canada)
Pierre-Aurèle Morin, MD (CCNMT)
Joanne Nixon (TTISS Ontario)
Andréanne Trottier (CCNMT, MSSS)
Jun Wu, MD, PhD (PHAC)
Matthew Yan, MD (CBS)
List of abbreviations
Transfusion Associated Circulatory Overload
Adverse Drug Reaction
Adverse Transfusion Reaction
Blood Establishment
Blood Safety Contribution Program
Canada Vigilance Program
Canadian Blood Services
Canadian Network for Public Health Intelligence
Canadian Transfusion Adverse Event Reporting Form
Comité consultatif national en médecine transfusionnelle (Québec transfusion medicine advisory committee)
Héma-Québec
Institut national de santé publique du Québec (Institute of public health of Québec)
Medical Device Incident
Ministère de la Santé et des Services sociaux (Québec Ministry of Health and Social Services)
National Advisory Committee on Blood and Blood Products
Provincial/Territorial
Public Health Agency of Canada
Québec Hemovigilance System
Rapport d’événement indésirable associé à la transfusion (Québec adverse transfusion reaction reporting system)
Red Blood Cell
Transfusion Error Surveillance System
Transfusion Medicine Laboratory
Transfusion Related Acute Lung Injury
Transfusion Transmitted Injuries Surveillance System
Summary of Revisions
Revision Date
Details

Accident – an unexpected event that is not attributable to a deviation from standard operating procedures or applicable laws and/or regulations that could compromise human safety or the safety of blood.^
Adverse Transfusion Reaction (ATR) – an undesirable and unintended response to the administration of blood (including blood components or blood products) that is considered to be definitely, probably, or possibly related to these products. The ATR may be classified as serious or non-serious (see definitions below).
Blood Components – a therapeutic constituent of human blood intended for transfusion into a recipient prepared by a blood operator.
• Blood Components include whole blood, red blood cells, granulocytes, platelets (including pathogen inactivated technology platelets manufactured by blood operators), plasma and cryoprecipitate.
Blood Establishments^ – a person/facility that conducts any of the following activities in respect of blood:
• Importation;
• Processing;
• Distribution;
• Transformation (e.g. irradiation, splitting, washing, pooling); or
• Transfusion.
Blood Operators – a subset of blood establishments that collect, process, and distribute blood. Canadian Blood Services (CBS) and Héma-Québec (HQ) are the only two blood operators in Canada.
Blood Products – a product derived from human plasma by a fractionation process and authorized by Health Canada (e.g. DIN-assigned blood product; also called plasma protein products, plasma derivatives, fractionated blood products). These are included within the definition of a drug in the context of Health Canada – Canada Vigilance Serious Adverse Drug Reaction Reporting.
• Blood Products with DIN numbers include immunoglobulins, albumin, coagulation factor concentrates, and solvent-detergent plasma.
Note – non-plasma derived therapies (e.g. recombinant coagulation factor concentrates and pharmaceutical hemostatic therapies) which may be available from blood operators are also included within the definition of a drug in the context of Health Canada – Canada Vigilance Serious Adverse Drug Reaction Reporting.
Blood Safety Contribution Program (BSCP) – a program within the Public Health Agency of Canada which supports surveillance activities for blood, cell, tissue, and organ related adverse events in an effort to maintain the safety of the Canadian health system. The BSCP includes the:
• Transfusion Transmitted Injuries Surveillance System (TTISS);
• Transfusion Error Surveillance System (TESS); and,
• Cells, Tissues, and Organs Surveillance System.
Visit the Blood Safety Contribution Program webpage for further detail.
Canada Vigilance Program (CVP) – Health Canada’s post-market surveillance program that collects and assesses reports of suspected adverse reactions to health products marketed in Canada.
Canadian Transfusion Adverse Event Reporting Form (CTAERF) – a standardized national form intended to be used by hospitals for reporting any adverse event resulting from an incident (error or accident) or transfusion reaction to the TTISS of the BSCP.
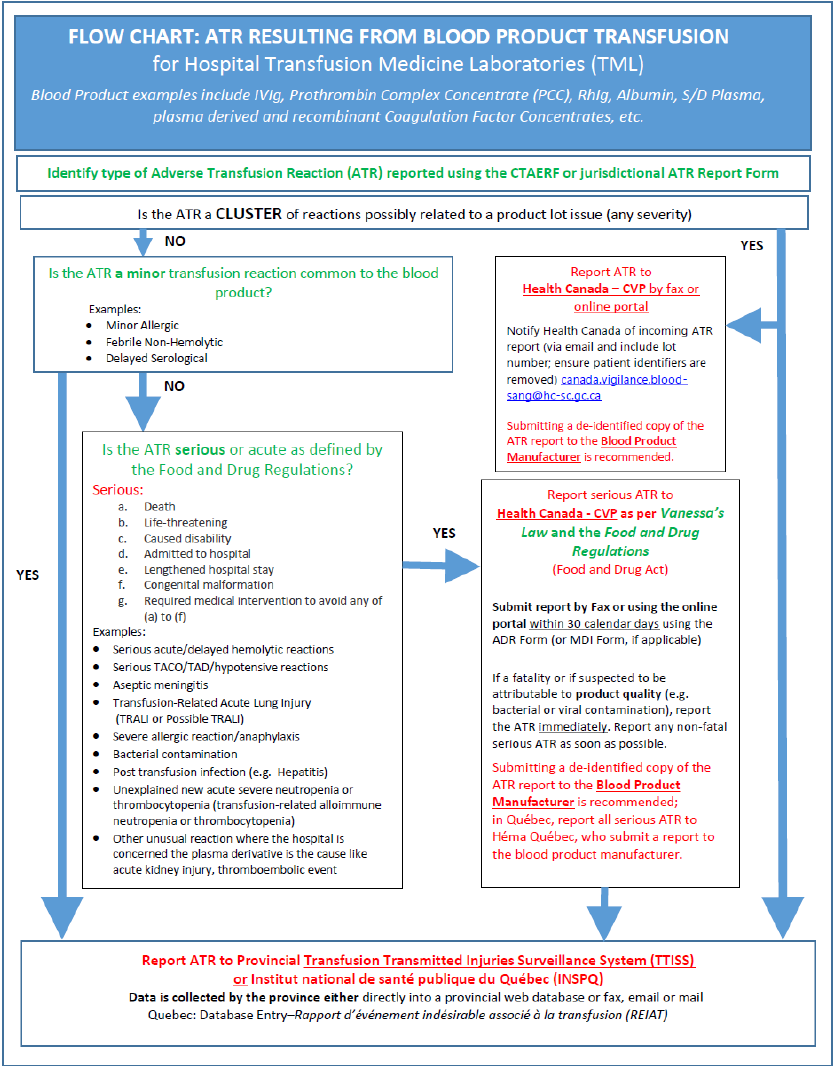
Cluster – an aggregation of cases grouped in place and time that are suspected to be greater than the expected number (e.g. increased frequency of expected or unexpected reactions of any severity suspected to be associated with a specific blood product lot number).
Note – this definition is from the textbook Field Epidemiology (2002), as there is currently no definition of cluster within Canadian ATR reporting organizations.
Error^ – a deviation from standard operating procedures or applicable laws or regulations that could compromise human safety or the safety of blood.
Hemovigilance – surveillance procedures covering the whole transfusion chain from collection of blood (components) to follow-up of its recipients. It assesses information on undesirable transfusion effects (reactions) to prevent their occurrence.
Note – this definition is from the International Society of Blood Transfusion – Haemovigilance Working Party, as there is currently no definition of hemovigilance within Canadian ATR reporting organizations.
Medical Device Incident (MDI)# – an incident related to a failure of a medical device or a deterioration in its effectiveness, or any inadequacy in its labelling or in its directions for use that has led to the death or a serious deterioration in the state of health of a patient, user, or other person, or could do so were it to recur.
See the Health Canada – CVP Guidance Document for further detail.
Non-Serious Adverse Transfusion Reaction – an adverse reaction to blood component or blood product transfusion that does not meet the definition of a serious adverse transfusion or drug reaction. This includes Grade 1 reactions as per the TTISS user manual.
Serious Adverse Transfusion Reaction – an adverse reaction that results in any of the following consequences for the recipient of transfused blood components or blood products:
• Requires in-patient hospitalization or its prolongation directly attributable to the event;
• Persistent or significant disability or incapacity;
• Necessitates medical or surgical intervention to preclude a persistent or significant disability or incapacity;
• Is life-threatening; or
• Death.
This includes Grades 2,3,4 reactions as per the TTISS user manual.
Serious Adverse Drug Reaction# – as defined in Part C, Division 1, subsection C.01.001(1.1) of the Food and Drug Regulations and for the purposes of the Food and Drugs Act, a noxious and unintended response to a drug (including a DIN-assigned blood product) that occurs at any dose and that requires:
• In-patient hospitalization;
• Prolongation of existing hospitalization;
• Causes congenital malformation;
• Results in persistent or significant disability or incapacity;
• Is life-threatening; or,
• Results in death.
Note – Besides the above seriousness criteria, Health Canada encourages hospitals to report adverse drug reactions, including those to blood products, that led to important medical events. Important medical events may not be immediately life-threatening or result in death or hospitalization, but may jeopardize the patient and may require intervention (e.g. emergency department or urgent care clinic visit) to prevent one of the serious outcomes listed above.
^ Denoted definitions are from the Government of Canada – Blood Regulations (SOR/2013-178)
# Denoted definitions are from the Government of Canada – Health Canada – Mandatory reporting of serious adverse drug reactions and medical device incidence by hospitals – Guidance Document (2019-06-26)
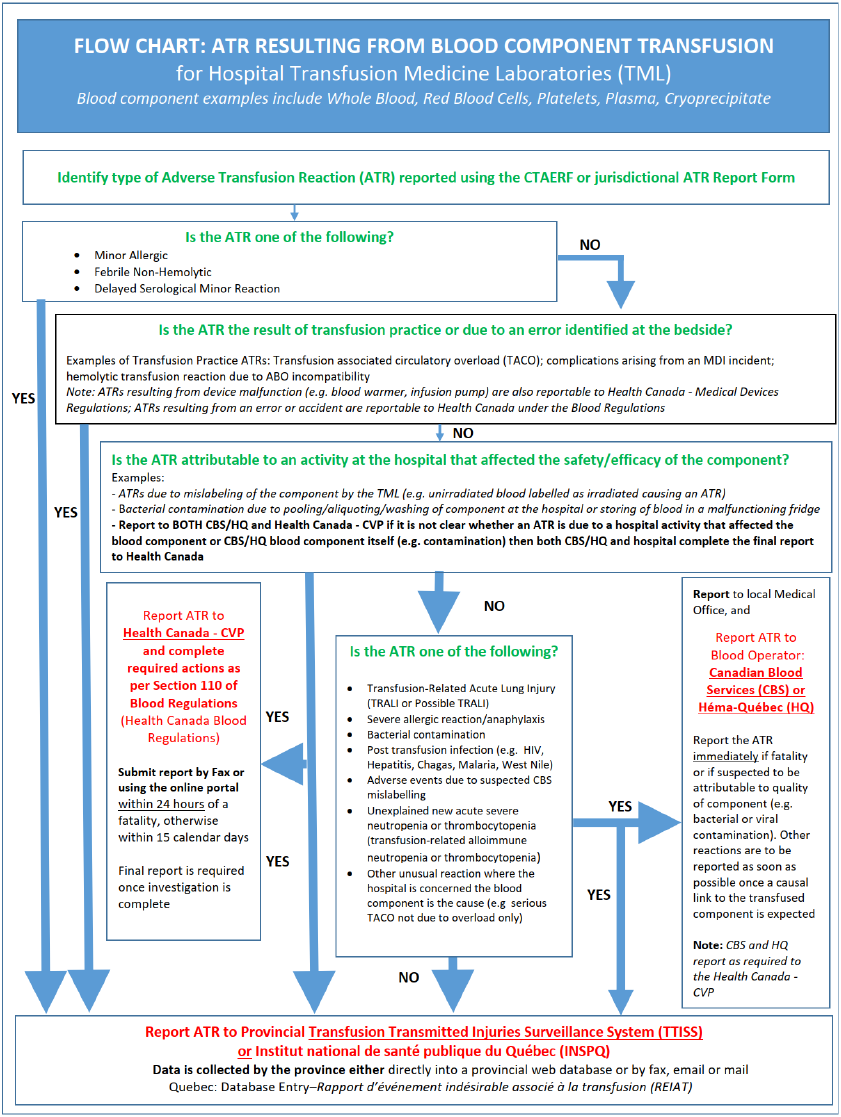
The purpose of this document is to provide hospitals with a resource summarizing adverse transfusion reaction (ATR) reporting processes in Canada. Details pertaining to transfusion related error and accident reporting is beyond the scope of this document.
Hemovigilance is important as a means of strengthening the overall safety of the blood system and transfusion for recipients. This requires hospitals to report suspected serious and/or unexpected ATRs because of a blood component (including red blood cells, platelets and plasma) or a blood product (e.g. intravenous immunoglobulin, prothrombin complex concentrates, Rh immunoglobulin, albumin, solvent-detergent plasma, etc.) transfusion, and subsequent investigation of the ATR cause and implementation of any outcome recommendations.
Following recognition and acute management of an ATR in a transfusion recipient, a formal notification of the ATR is submitted by the clinical team to the transfusion medicine laboratory (TML; also known as a blood bank), as per local policy and procedure. Once the ATR notification is received, healthcare professionals within the hospital investigate the suspected ATR, as outlined by provincial/territorial (P/T) processes. Healthcare professionals responsible for investigation of the ATR circumstances and subsequent summary report submission to required authorities may include transfusion safety officers or transfusion safety nurses, medical laboratory technologists, quality assurance officers or transfusion medicine physicians within hospital transfusion medicine services.
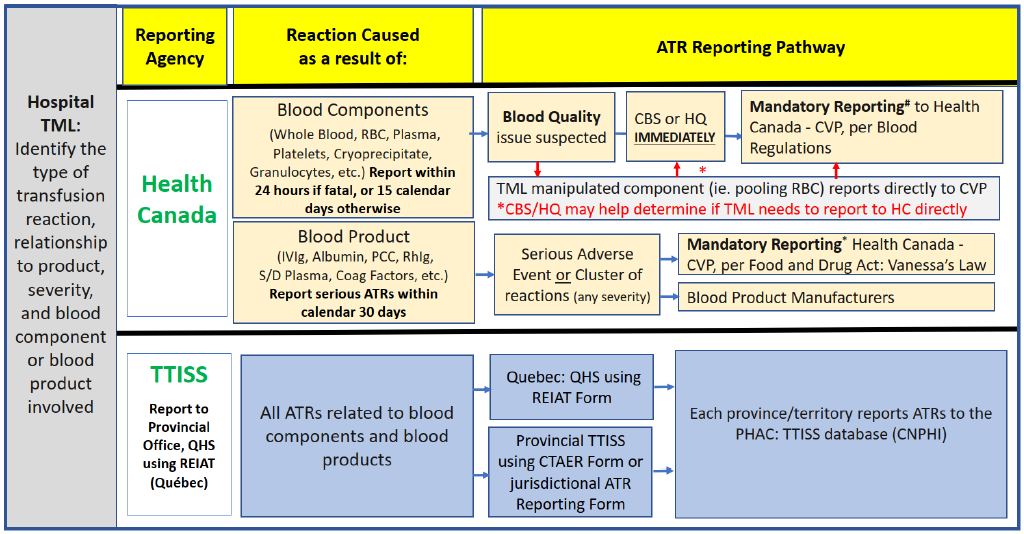
In Canada, there are two national health organizations which receive transfusion adverse reaction data as submitted by hospital transfusion medicine services:
- Health Canada – the Canada Vigilance Program (CVP), which receives reports from Canadian blood establishments, including blood operators and hospitals, in accordance with requirements of the federal Blood Regulations and the Food and Drug Regulations.
- The Public Health Agency of Canada (PHAC), which manages the Transfusion Transmitted Injuries Surveillance System (TTISS).
PHAC – TTISS and Health Canada – CVP are part of Canada’s Health Portfolio, but they each have different purposes and reporting systems.
At present, Health Canada – CVP and PHAC – TTISS do not share common database systems, as the data for both programs come from different sources. Health Canada – CVP receives reports of serious adverse reactions primarily through blood operators (Canadian Blood Services (CBS)/Héma-Québec (HQ)) under the Blood Regulations, whereas the TTISS receives reports of adverse reactions from P/T blood transfusion facilities.
2.1 Health Canada and the Canada Vigilance Program (CVP)
Hemovigilance is mandated federally by Health Canada, a regulatory body, through both the Blood Regulations and Food and Drug Regulations on behalf of the federal Minister of Health.
The CVP is a post-market surveillance program managed by Health Canada. The CVP collects and assesses reports of suspected adverse reactions to health products (including investigations of ATRs).
Health professionals and consumers report voluntarily while manufacturers, distributors, and hospitals are regulated parties obligated to report under the Food and Drug Regulations and Blood Regulations.
A key goal of Health Canada – CVP is to ensure the safety of the blood supply through the collection and analysis of reported ATRs related to the quality of blood component or blood product. More specifically, ATRs related to:
- Blood components are primarily reported to the Health Canada - CVP by blood operators (CBS/HQ); and
- Blood products are reported to the Health Canada - CVP by hospitals.
Note: The reporting responsibility falls to the TML personnel within hospitals. Health Canada regulates the hospitals which transfuse blood, not the regional health authorities.
2.1.1 Blood Regulations
The Blood Regulations were established to promote and protect the safety of Canada’s blood supply used for transfusion or for further manufacture into a drug for human use. The Blood Regulations are administered by the Health Products and Food Branch of Health Canada (which is a distinct entity from PHAC). They contain requirements for human safety and the safety of blood with respect to the following activities related to human blood and blood components for transfusion and processing*:
- Transforming (e.g. irradiation, splitting, washing, pooling);
- Labelling;
- Storing;
- Record keeping;
- Importing;
- Distributing; and,
- Error, accident, and adverse reaction investigation and reporting.
*Note that processing includes donor suitability assessment, collection, testing, and blood component preparation.
The Blood Regulations came into force in October 2014. All transfusing establishments are expected to identify and report serious ATRs. The organization performing the attributable activity (or activities) is responsible for reporting to Health Canada – CVP and completing the full investigation. In most cases this responsibility lies with the blood operators: CBS or HQ. Rarely, hospital blood banks may be the investigating establishment especially if they carried out a transformation activity with respect to the implicated blood.
If the serious ATR is determined to be attributable to the quality or safety of the blood component received from the blood operator (either HQ in Québec, or CBS in all other provinces/territories), the hospital must immediately submit a report to the blood operator, who then becomes responsible for the investigation and reporting to Health Canada – CVP. Reporting must occur within 24 hours once the reporter has reasonable grounds to suspect a fatality is related to the quality and safety of the transfused product, or 15 calendar days otherwise. See Appendix 1 and Appendix 2A for details.
Note: the reporting of errors and accidents to blood components are beyond the scope of this document. For more information on Errors and Accidents, please refer to Sections 103 to 108 in the Guidance Document: Blood Regulations.
2.1.2 Mandatory Reporting and the Food and Drug Act: Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law)
The Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law) received Royal Assent on November 6, 2014 and came into force on December 16, 2019.
The Food and Drug Act was amended by Vanessa’s Law, which improved Health Canada’s ability to collect post-market safety information and take appropriate action when a serious health risk is identified. Several key amendments were made to the Food and Drug Act, including the introduction of mandatory reporting of serious adverse drug reactions (ADRs) and medical device incidents (MDIs) by healthcare institutions.
As of December 16, 2019, hospitals became newly regulated parties who must report serious ADRs and MDIs to Health Canada – CVP within 30 calendar days of documentation within the hospital.
The mandatory reporting requirements apply, but are not limited to, the following therapeutic products regulated under the Food and Drug Regulations and the Medical Device Regulations:
- Biologic drugs, which include fractionated blood products (plasma proteins);
- Drugs, including non-plasma derived products available from blood operators; and,
- Medical devices, more specifically medical device equipment failures (also called MDIs) related to blood transfusion procedures. An example of medical devices includes blood warmers and blood infusion devices.
The Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals - Guidance Document may be consulted for more information, namely:
- Section 3 Roles and responsibilities
- All hospitals that are regulated through P/T legislation, as well as those operated by the federal government that provide health services to patients, are required to fulfil requirements of mandatory reporting. Reporting is the responsibility of the hospital facility, rather than the individual health care professionals or specific hospital departments. While healthcare professionals play an important role in recognizing and documenting serious ADRs, ATRs and MDIs, it is the hospital that is responsible for determining clear internal roles and responsibilities for its employees or contract workers/companies/blood banks in complying with the mandatory reporting obligations. Hospital staff should be familiar with serious ATR, ADR and MDI reporting procedures within their local institutions.
- Section 5.2 Examples of serious adverse drug reaction, including fractionated blood products (plasma proteins).
- Section 6 Information requirements for Serious Adverse Drug Reaction and Medical Device Incident reports
- There are required elements which need to be included in serious adverse drug (blood product) reaction and MDI reports. For complete details regarding the required data elements, please see Section 6.
Note: Hospitals are encouraged to also send a copy of the report to the manufacturer, who is required by the Food and Drug Regulations to report serious ADRs to Health Canada. See Appendix 1 and Appendix 2B for details.
2.2 The Public Health Agency of Canada (PHAC): Transfusion Transmitted Injuries Surveillance System (TTISS)
In response to the 1997 Report of the Commission of Inquiry on the Blood System in Canada, the federal government launched a series of initiatives and provided funding to improve the safety of Canada’s blood system. The PHAC Blood Safety Contribution Program (BSCP) supports the development and/or enhancement of P/T systems that monitor adverse events associated with transfusion and transplantation. The TTISS was launched in 1999 as one of the three surveillance networks of the BSCP.
The TTISS is a national surveillance system for reporting of adverse reactions to the blood, blood components, or blood products (plasma derivatives). The main purpose of the TTISS is to monitor ATR incident rates and trends and gain better knowledge of the risk of ATRs occurring in Canada. Reporting to the TTISS is voluntary.
TTISS is designed to collect all ATRs which occur in a transfusing establishment, regardless of reaction severity and the relationship of the reaction to blood quality. Each province or territory collects ATR reports which are entered by local staff into the national TTISS database within a central web-based system of the PHAC known as the Canadian Network for Public Health Intelligence (CNPHI). However, data submitted to the TTISS within the last decade have predominantly included only moderate and severe (serious) reactions; some provinces have not consistently included minor ATR (e.g. febrile non-hemolytic, allergic) reaction data with their reports.
The TTISS was initiated as a pilot project in four provinces; British Columbia, Québec, Nova Scotia and Prince Edward Island, and included ATR data for the period of 2001-2002. Following the demonstrated success of this pilot project, the TTISS has since been a national system since 2012, with all provinces and territories participating in TTISS reporting. Québec continues to maintain a comprehensive provincial Québec Hemovigilance System (QHS). Hospitals in Québec report their ATR data to the QHS, which then reports all definite, probable, and possible ATRs yearly to the TTISS database. As of 2020, the TTISS participation rate of hospitals providing transfusion services in Canada was more than 95%.
National TTISS summary reports are available online on the PHAC Publications - Drugs and Health Products webpage, under the Blood Products subheading.
2.2.1 Reporting ATRs to the TTISS
The TTISS was initiated by the federal government, as a recommendation of the Krever Commission, to improve the safety of the Canadian blood system.
A Canadian Transfusion Adverse Event Reporting Form (CTAERF) and a TTISS User’s Manual have been developed by a National TTISS Working Group consisting of P/T representatives, manufacturers of blood components, Health Canada, and PHAC personnel. The TTISS User’s Manual is to be used as a resource for completing the CTAERF or TTISS database.
All provinces and territories are required to upload their annual ATR data into the CNPHI system within six months of the end of the calendar year. For example, January-December 2020 data is to be reported by June/July 2021, and so on for the next successive years.
A working group with experts in their respective provinces and territories review preliminary findings of their annual data to identify in case of any errors or omissions. After data verification, TTISS preliminary reports are generated at a national level.
As a result of hospital-based human resources limitations, it has not been feasible for all provinces and territories to provide comprehensive data on Grade 1 minor reactions, including febrile non-hemolytic reaction and minor allergic reaction, as well as delayed serological transfusion reaction (new alloantibodies). To assure uniformity of ATR reporting among the provinces and territories, and to facilitate international comparison of ATR rates, PHAC continues to recommend that all ATRs be reported into the CNPHI database.
Details regarding the signs and symptoms of all ATRs are available in the CNPHI database as well as in the TTISS User’s Manual.
To maintain consistency of ATR reporting in Canada, the use of the TTISS User’s Manual ATR definitions is encouraged. The PHAC recognizes that the CATERF and TTISS User’s Manual were developed in 2007.
The transfusion community is advocating for an interim update of transfusion reaction definitions for ATRs to reflect formal definition updates in the literature. Should an interim update or a revised TTISS User’s Manual become available, these will be posted on the PHAC website. PHAC will communicate the release of these resources with stakeholders as soon as they are published.
Within Québec, the Institut national de santé publique du Québec (INSPQ) has published Guidance on Declaring Adverse Transfusion Reactions, which includes updated ATR definitions reflecting the current literature. The QHS encourages use of the INSPQ guidance document within Québec; at present, this document is only available in French.
2.3 Canada’s Blood Operators: ATR Reporting to CBS or HQ
Requirements for reporting ATRs to blood components to blood operators (CBS and HQ) are outlined in the Blood Regulations. This includes serious or unexpected ATRs where there is concern for the safety of the blood component and/or initial investigations suggest possible attribution to an activity completed by the blood operator. Blood operators will conduct an investigation and report applicable ATRs directly to Health Canada. A separate report from the hospital to Health Canada – CVP is not necessary unless it is attributed to a regulated activity done at the hospital as outlined in Section 2.1.
At minimum, initial reports to the blood operator should include the suspected reaction type and implicated component unit numbers. Additional information as described in the Guidance Document: Blood Regulations from the hospital investigation, including testing results and relevant clinical recipient information, should be forwarded to the blood supplier as soon as they are available. Any local or provincial ATR form can be used for reporting an ATR to the blood operator.
Examples of reportable ATRs to the blood operator include:
- Severe allergic reaction or anaphylaxis;
- Transfusion Associated Lung Injury (TRALI);
- Transfusion-Associated Graft vs Host Disease;
- Bacterial contamination;
- Transfusion-transmitted infections; and,
- Hemolytic Transfusion Reactions directly attributable to product labelling (e.g. phenotyping error, low titer anti-A/anti-B designation).
In the case of an ATR related to blood components collected by another blood operator outside of your jurisdiction, please contact your local blood operator for guidance.
For specific detail regarding ATR reporting to Canada’s blood operators, please consult the following resources:
- Canadian Provinces (except Québec): A Guide to Reporting Adverse Transfusion Reactions
- Guidance Document: Blood Regulations
- Québec: Publication de l’Institut national de santé publique du Québec - Guide de déclaration des événements indésirables associés à la transfusion de produits sanguins
| Reporting Authority | Form |
|---|---|
PHAC-TTISS - ATRs with sign-symptoms along with relationship/ severity/outcome during or after blood transfusions. | Fax the a de-identified provincial/jurisdictional ATR report form or a completed Canadian Transfusion Adverse Event Reporting Form (CTAERF) to your local or P/T TTISS designate for data entry into CNPHI.
|
Health Canada - CVP - Serious or unexpected ATRs related to quality/ | To report serious or unexpected ATRs related to quality/safety of blood component:
|
Health Canada - CVP - Trends/clusters of non-serious (minor) ATRs to blood products. | To report trends/clusters of non-serious (minor) ATRs to blood products:
Note: for general blood-related inquiries or if you suspect lot-associated issues (such as potential clusters of serious and non-serious reactions), you may wish to notify canada.vigilance.blood-sang@hc-sc.gc.ca prior to submitting the report form(s). Please do not send copies of completed reports containing patient information to this email address. |
Health Canada – CVP - Serious ATR to blood product; | To report serious ATRs to blood product and MDIs:
|
Health Canada - Errors and Accidents involving blood components (under the Blood Regulations). | For Errors and Accidents involving blood components (under the Blood Regulations):
Notes:
|
| Canadian Blood Services |
Fax completed form to your local CBS Distribution Operations Centre. |
| Héma-Québec |
|
| Product Manufacturer |
A de-identified provincial/jurisdictional ATR report form. Note: A list of product manufacturer fax and phone numbers are available at https://www.blood.ca/. To obtain the contact information you must follow these steps:
|
1When submitting a provincial ATR form to CBS or the CVP, remove patient identifiers so that only the patient’s date of birth, sex, and date of transfusion are reported. 2The online reporting application connects you to the correct form to report to the Health Canada – CVP; select the appropriate suspected product (drug or medical device) then the reporter that best describes you (i.e. healthcare professional or facility). |
For a comprehensive list of contact details for P/T offices and national ATR reporting organizations, please see the Supplement to this document.
The following link contains an interactive portal, which may be used to assist with ATR reporting decisions, complimenting the Appendices at the end of this document: https://redcap.link/CanadianWHOtoreportTransfusionReactionAlgorithm
In his 1997 report, Justice Krever emphasized the importance of surveillance and tracking of blood components and products for transfusion, referring to the concept of vein-to-vein management of blood.
The Transfusion Medicine community in Canada recognizes the continuing need for a hemovigilance system which not only facilitates collection of ATR data, but also provides more timely reporting of national ATR rates. As demonstrated from the example provided by the annual reports published by the United Kingdom Serious Hazards of Transfusion (SHOT) hemovigilance scheme, this information raises the awareness of transfusion safety and provide recommendations to enhance patient care and safety with respect to blood component and blood product transfusion.
Elements of a well-functioning hemovigilance system should include:
- Cross jurisdictional collaboration and data sharing;
- Timely and accurate ATR data reporting;
- Well-resourced data management systems and information repositories;
- Unified electronic system of national ATR reporting to remove duplication of reporting in multiple systems and to enhance hemovigilance data integrity; and,
- Consistent mechanisms to collect blood component and blood product issue data from transfusion medicine services, to ensure accurate denominator data capture for ATR rate reporting.
The recently published BSCP audit report entitled Evaluation of the Public Health Agency of Canada’s Blood Safety Contribution Program 2017-18 to 2021-22 includes recommendations and a documented action plan to help strengthen the BSCP and its important role in Canadian hemovigilance.
References
Canada Vigilance Program https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/canada-vigilance-program.html
Guidance Document: Blood Regulations https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/blood-regulations/guidance-document-blood-regulations-1.html
International Society for Blood Transfusion: Haemovigilance Working Party. https://www.isbtweb.org/isbt-working-parties/haemovigilance.html
INSPQ Guide (January 2023): https://www.inspq.qc.ca/publications/3355
Medical Devices Regulations: https://laws-lois.justice.gc.ca/eng/regulations/sor-98-282/
Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals - Guidance document https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting/mandatory-hospital-reporting/drugs-devices/guidance.html
Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law) https://laws-lois.justice.gc.ca/eng/annualstatutes/2014_24/page-1.html
Public Health Agency of Canada Transfusion Transmitted Injuries Surveillance System, User’s Manual, Version 3. 2007.
Gregg MB, ed. Field Epidemiology. 2nd ed. New York, NY: Oxford University Press. 2002.
Yan, M. A Guide to Reporting Adverse Transfusion Reactions. January 2020. Canadian Blood Services. https://professionaleducation.blood.ca/en/transfusion/publications/adverse-reactions-reporting
Appendices
Reporting of Adverse Recipient Transfusion Reactions by Hospitals to the Health Canada - Canada Vigilance Program and Public Health Agency of Canada -- Transfusion Transmitted Injuries Surveillance System (TTISS).
The following link contains an interactive portal, which may be used to assist with ATR reporting decisions: https://redcap.link/CanadianWHOtoreportTransfusionReactionAlgorithm