NAC Statement on Perioperative Autologous and Matched Donations
Matthew Yan, MD
List of abbreviations
Patient Blood Management
Transfusion Associated Circulatory Overload
Canadian Blood Services
Hepatitis B
Hepatitis C
Human Immunodeficiency Virus
Perioperative Autologous Blood Donations
Red Blood Cell
Transfusion Related Acute Lung Injury
Transfusion-Associated Graft-versus-Host Disease
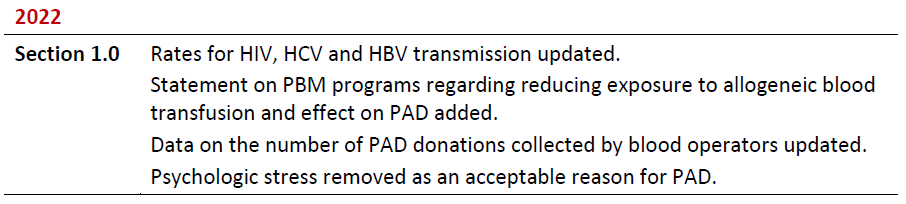
Summary of Revisions
Revision Date
Details

1.1 Perioperative Autologous Blood Donations
Perioperative autologous blood donations (PAD), as a strategy to avoid allogeneic red blood cell transfusion, has been in decline over the last 15 years due to a decrease in demand from physicians and patients. PAD was originally promoted in the 1980s and subsequently by the Krever Royal Commission as an important mechanism to decrease exposure to allogeneic transfusions and the associated risks of transfusion, particularly viral transmission. This recommendation appears to no longer be relevant due to current transfusion practice. Over the past three decades, the risk of transfusion-transmitted viral infection has been dramatically reduced due to advancements in donor testing, screening and pathogen reduction modalities. The residual risk estimates for viral transmission following an allogeneic transfusion are very low at 1 in 12.9 million donations for HIV (human immunodeficiency virus), 1 in 27.1 million donations for hepatitis C (HCV), and 1 in 2.0 million donations for hepatitis B (HBV). Additionally, there has been increased focus on patient blood management (PBM) programs to decrease allogeneic transfusions through evidence-based practice including preoperative anemia optimization and restrictive transfusion practices. Consequently, for Canadian blood operators, the number of autologous units collected has decreased from 5682 units in 2007 to 4 units in 2021-22. In 2020, there were no autologous units collected by hospital-based programs. This dramatic reduction reflects the trends for PAD across most jurisdictions in North and South America, and Europe, and is at least partially related to the recognition of the current safety of allogeneic blood transfusions.
With the reduction in the risk of viral transmission through blood transfusion, the impetus for PAD has significantly reduced. While some potential benefits of autologous red blood cell donation remain, these are offset by potential risks and adverse effects associated with PAD. If allogeneic transfusions are avoided, PAD can eliminate risk of alloimmunization in addition to eliminating the risk of viral transmission. However, PAD increases the risk of perioperative anemia, which may be associated with increased morbidity. As PAD often results in decreased hemoglobin levels at the time of surgery, this can result in higher rates of transfusion for any blood product (autologous + allogeneic). Autologous red blood cell (RBC) transfusions may still be associated with transfusion complications including bacterial infections, transfusion-associated circulatory overload (TACO) and, perhaps most importantly, transfusion of an incorrect unit (either for the autologous donor or another patient receiving the autologous unit in error). Additionally, PAD can result in lower pre and postoperative hemoglobin levels, and there is increased recognition of perioperative anemia being associated with poorer postoperative outcomes and increased healthcare costs.
While there has been a marked reduction in PAD, there was previously a very high discard rate for the autologous red blood cell units since these units could not be crossed over into the regular blood supply. In Canada, the discard rate for PAD units was 70%. This represents a waste of valuable healthcare resources for the collection and storage of RBCs that are never used. The high discard rate is particularly important as the collection of these PAD units may result in lower hemoglobin levels post-operatively. Thus, PAD can result in possible harm to patient with absolutely no benefit for 70% of PAD units collected.
Given the reduction in risks associated allogeneic transfusions and the potential adverse effects associated with PAD, recommendations from various international organization have suggested that PAD should not be routinely performed. Rather it should be limited to very specific clinical scenarios where there is clear benefit to patients (i.e. patients with antibodies to high frequency antigens who are undergoing surgery and would be difficult to support with the regular allogeneic blood supply).
1.2 Matched Donations
A matched donation is blood collected from a donor for a specified recipient of their choice. Canadian Blood Services (CBS) has previously allowed for matched donations through their Directed Donation Program collected from an ABO-compatible parent or a legal guardian to be transfused to their minor child1,2. A matched donation could be either red cells or apheresis platelets. CBS has not collected any products through their Directed Donation Program since 20193. Individuals providing matched donations through the Directed Donation Program must meet all the regular blood donation criteria and undergo all routine tests for a regular blood donation. However, blood that is collected through the Directed Donation Program cannot be used for another recipient as they are considered potentially higher risk and, therefore, the units must be discarded if they are not used.
Matched donations, particularly from family members, may be associated with increased risk to the recipient. A transfusion from a first-degree relative can cause transfusion-associated graft versus host disease (TA-GvHD), which is a severe and potentially fatal reaction. To reduce the risk of this reaction, the matched donations must be irradiated, which affects the product quality and will reduce the shelf-life. A case-control study also linked matched donations from maternal donors to their child with increased rates of transfusion-related acute lung injury (TRALI) reaction7. Frequently matched donors are also first-time blood donors who have higher rates of transmissible disease testing as compared to repeat blood donors. Additionally matched donors may feel additional pressure to donate and may not be forthcoming about risk factors which increase the risk of an adverse transfusion reaction. For all the above reasons, matched donations are associated with increased risk of adverse reactions, without any evidence of benefit for the recipient unless there is need for rare blood type that could not be otherwise supported through the regular allogeneic blood program.
For patients who require matched blood to be collected from a family member due to the presence of an antibody to a high frequency antigen (RBCs) or HLA antibody (platelets), the family members are encouraged to become a regular blood donor. These donors would need to meet all the criteria for regular blood donation and undergo all routine screening blood tests. Their blood would then be collected and used if required for their family member. An additional benefit of collecting matched donations through the regular allogeneic system, is the ability to freeze any unused red cells as part of the CBS Rare Blood Disorder Program such that the units could be used for a future blood recipient (including the donor themselves) requiring the specific phenotype.
- Given the increased safety of allogeneic transfusions and the potential adverse effects associated with PAD, the National Advisory Committee on Blood and Blood Products recommends that PAD not be routinely performed as a strategy to reduce allogeneic blood transfusions.
- Given the increased safety of allogeneic transfusions, the potential increased risk associated with directed blood donation and the increased cost associated with directed blood donations, the National Advisory Committee on Blood and Blood Products recommends that directed donations not be used as a strategy for individuals to avoid receiving blood transfusion from the regular CBS allogeneic blood supply.
- PAD should be restricted to patients who meet both of the following requirements:
- Undergoing surgery with high risk of transfusion;
- The presence of antibody(ies) to either high frequency antigens or associated with rare phenotypes in whom it is not possible to support the patient’s transfusion requirements with the regular allogeneic blood supply.
- All patients being considered for PAD must be discussed with the hospital transfusion medicine physician.
- For patients meeting the exceptional criteria for PAD, it is critical that all PAD units are collected 3-4 weeks prior to surgery to minimize the risks of iatrogenically induced perioperative anemia. In these patients, perioperative iron replacement should be undertaken as appropriate.
- Matched donations are only indicated for patients with:
- Antibody(ies) to either high frequency antigens or associated with rare phenotypes, or
- Rare HLA antibodies (+/- rare HLA typing).
Since all matched donors must meet all the criteria for regular donation, there is no need for a separate Directed Donor Program. These donations can be managed through the regular allogeneic donation process including the CBS Rare Donor and HLA Apheresis Platelet Programs. Importantly, for matched red cells donated through the regular allogeneic program, any unused red cell units can then be incorporated into the CBS Rare Donor Program for future patients requiring these rare phenotypes.
References
- Foster T, Yan M. Preoperative autologous donation. In: Clarke G, Chargé S, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2020 [cited 2022 10 31]. Chapter 16
- Blood Notes: Preoperative autologous blood donations [https://www.blood.ca/sites/default/files/PAD_and_Autologous.pdf]. Ottawa: Canadian Blood Services, 2018. . [cited 2023 03 25].
- Top Abstracts from the 2020 Canadian Society for Transfusion Medicine (CSTM) Annual Meeting. Transfusion Medicine Reviews 2021;35:56–57
- Vassallo R, Goldman M, Germain M, Lozano M for the BEST Collaborative. Preoperative Autologous Blood Donation: Waning Indications in an Era of Improved Blood Safety. Transfusion Medicine Reviews. 2015;29:268–275
- Bouton FE, James, V. (British Committee for Standards in Haematology, Transfusion Task Force). Guidelines for policies on alternatives to allogeneic blood transfusion. 1. Predeposit autologous blood donation and transfusion. Transfusion Medicine. 2007;17:354–365.
- Leal-Noval SR,Muñoz M, Asuero M, Contreras E, García-Erce JA, Llau JV, et al, Spanish Expert Panel on Alternatives to Allogeneic Blood Transfusion. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document.”. Blood Transfusion. 2013;11:585–610.
- Dunbar N, Cooke M, Diab M, Toy P. Transfusion-related acute lung injury after transfusion of maternal blood: a case-control study. Spine. 2010;35:E1322-7
