NAC Position Statement on Patient Blood Management
Charles Musuka, MBChB
Katerina Pavenski, MD
Tanya Petraszko, MD
Taher Rad, MD
Lucinda Whitman, MD
Harleen Kahlon (ON)
List of abbreviations
Erythropoietin Stimulating Agent
Information Technology
Patient Blood Management
Transfusion Associated Circulatory Overload
Gastrointestinal
Intravenous Iron
National Advisory Committee on Blood and Blood Products
Patient Blood Management (PBM) is the timely application of evidence-based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis and minimize blood loss in an effort to improve patient outcome.1 Framework for PBM programs may vary whether in a surgical setting (pre-operative, intra-operative, and post-operative)2 or by goal (stop/minimize blood loss and diagnostic phlebotomy, diagnose and treat coagulopathy, manage anemia, and improve tolerance of anemia)3. The aim, regardless of framework, is to improve patient outcomes and make patients the center of care.
PBM programs consistently demonstrate reduced transfusion utilization and cost avoidance4; and they frequently demonstrate reduced hospital length of stay, reduced morbidity, reduced mortality, and overall improved patient outcomes5. These benefits are in contrast to the increased recognition that ignoring anemia or utilizing transfusion to treat anemia may increase complications including: infection6, thrombosis7, stroke/myocardial infarction8, transfusion related immunomodulation (which may increase cancer progression and/or recurrence)9,10, and transfusion reactions such as transfusion associated circulatory overload (TACO) and transfusion associated lung injury (TRALI)11.
In many institutions, patient blood management is the standard of care12. The World Health Organization issued a resolution in 2010 to improve patient safety by implementing patient blood management programs,13 and in 2021 issued an updated policy brief calling for an urgent need to implement patient blood management14. Specifically, they state, “All Member States should act quickly through their ministry or department of health to adopt their national PBM policy, install the necessary governance, and reallocate resources to improve the population health status and individual patient outcomes while reducing overall health care expenditures.” World-wide, there has been country-wide implementation in Austria, the Netherlands15, and Western Australia16. While there are some references to PBM by Canadian Blood Services17, and principles may be found in the NAC blood shortages document18, more widespread application across the healthcare spectrum is necessary to improve health care provision and blood utilization in Canada. Many processes need to be aligned, and it is short sighted to attempt to perfect anemia management once a blood shortage occurs. PBM is interdisciplinary and goes beyond transfusion; cooperation and education amongst nursing, physicians, administration, pharmacy, laboratory, and perfusion staff is necessary. PBM should also reflect the patient’s medical history, preferences and values.
Successful Patient Blood Management Programs include the following19:
Education - Many medical practitioners are accustomed to certain practice behaviors that become outdated over time20. An example is the transfusion of two units of red blood cells in response to anemia. This has become a focus for Choosing Wisely’s, “Why Use Two When One Will Do” campaign21. Likewise, transfusion thresholds have steadily decreased over time in response to higher quality evidence advocating for more restrictive thresholds. Unfortunately, implementation of this evidence has been slow and heterogeneous. Finally, while the evidence has supported the use of intravenous iron (IV iron) and erythropoietin stimulating agents (ESA) to reduce transfusion needs22, their implementation into practice lags. The reasons for this delay are many, but lack of medical practitioner education on these transfusion alternatives represents a significant barrier to their adoption.
Physician resources - Many programs have a physician director for managing referrals and intervention of complex forms of anemia. A lack of physician education and assigned responsibility for managing anemia results in patients missing opportunities for optimization and better outcomes prior to and during their hospital admission.
Nursing resources - Nursing care forms the backbone of patient blood management programs in terms of direct patient care. A dedicated nursing position ensures continuity of care. Currently in Canada, the Ontario Nurse Transfusion Coordinator (ONTraC) program exemplifies the important role of nursing in patient blood management23. Not all sites in Ontario have this program, and most sites outside of Ontario have no formal program. The ONTraC program has one of the best toolkits available for hospital implementation24.
Administrative resources - Scheduling patient consultations, interventions, and follow-up is also necessary to maintain patient flow in their care journey. Developing and employing data tracking to ensure quality metrics are achieved is also important.
Physical resources - Some aspects of patient blood management include administration of intravenous and subcutaneous injections, which require patient assessment and monitoring. The best example is intravenous iron. Depending on the IV iron formulation chosen, total chair time to restore iron stores may be as short as 1 hour or up to 10 hours25. This requires monitored space to accommodate patients undergoing treatment, as well as nursing and physician resources to staff and supervise administration.
Timing to critical events - A robust system identifies patients well in advance of their surgical or obstetrical delivery date. To utilize low-cost oral iron requires nearly three months on average to restore iron in deficient patients. Most surgical systems do not notify patients or practitioners of upcoming dates with enough time to utilize oral supplements. Peak effect for IV iron and ESA still requires several weeks of planning to optimize anemia and limit transfusion, although there is some evidence that even a single day results in some improvement26.
Pharmacy resources - Pharmacy needs to be actively engaged as most budgets are isolated from one another. Labile blood components are funded by a transfusion budget, but adjunctive therapy including IV iron and ESA are funded by a pharmacy budget through exceptional drug status, private insurance, or a patients’ own funds. The savings are a result of reduced transfusion costs, activity costs (compatibility testing, inventory management), and reduced hospital costs when the hospital stay is reduced. Conversely, treating anemia without transfusion often increases the pharmaceutical cost. As a net equation, PBM reduces overall system costs. Therefore, pharmacists need to be actively engaged in ensuring appropriate resource management and reimbursement, and to consider the risks and benefits of labile blood components as compared to the risks and benefits of a medication.
Medication funding - Medications specifically shown to reduce transfusion or treat anemia should be considered. Specifically, antifibrinolytics (tranexamic acid)27, IV iron (ferric gluconate, iron sucrose, ferric derisomaltose)28, and ESAs (Darbopoetin, Epoetin alpha)29 have a significant transfusion-sparing effect and should be publicly funded.
Laboratory Resources - Laboratory clinicians can often improve diagnostic accuracy and timeliness to assess patient response to therapy. An example would be measuring reticulocyte markers (early red blood cell production) in response to oral iron to assess the need to progress to IV iron or add an ESA. Many laboratories also oversee point of care testing which may reduce the volume of blood lost by patients during diagnosis. Similarly, laboratories can strive to reduce unnecessary diagnostic phlebotomy, duplicate or mislabeled specimens by use of positive patient identification, and use of smaller tubes or those with less vacuum to collect blood samples.
IT support - Data drives decisions and informs future management. A robust system to capture pre and post implementation allows evaluation of value-added patient blood management and helps drive future clinical decisions. Implementing clinical decision support for physician order entry has been shown to reduce unnecessary transfusions30.
Perfusion resources - Not every hospital will have perfusion personnel, but where present and in appropriate situations, perfusionists provide cell saver support in massive hemorrhage and high-risk operations. They are an integral part of providing safe cardiovascular surgical care and introducing measures to reduce the risk of transfusion in cardiac surgery.
A. Systemic Recommendations
1. All hospitals should work with their provincial Ministry of Health and health sector partners to implement PBM as a best practice that improves patient outcomes and system efficacy. A multimodal perioperative PBM program should be instituted in all surgical programs to address pre-operative, intra-operative, and postoperative anemia. The resources outlined in Section 2, which are required for successful implementation of patient blood management, should be provided on a site-by-site basis with consideration of clinical need and system resources.
2. Provinces should encourage hospitals to participate in initiatives including Choosing Wisely and Using Blood Wisely which align with patient blood management principles. Providing order sets, and screening for single unit transfusions and restrictive transfusion thresholds (less than 70g/L in all patients except those at risk of ischemia where the threshold is less than 80g/L) reduces blood utilization without adding cost.
3. Educational resources should foster the development of local patient blood management leaders and champions. All health care practitioners should be aware that anemia (hemoglobin less than 130g/L in peri-operative patients) increases morbidity and mortality. This is true of pre-existing anemia and hospital acquired anemia. Hospitals should recognize that routine or avoidable diagnostic blood draws can result in hospital acquired anemia and prolong patient recovery. Stopping and minimizing blood loss requires interdisciplinary efforts and should be a primary pillar for PBM programs.
B. Clinical Recommendations
4. All patients undergoing surgery or delivery should be screened for anemia at least 6 weeks prior to their anticipated surgical or delivery date.
5. Appropriate referral to a specialist for investigation and management of underlying conditions is recommended and may include gastroenterology, gynecology, hematology, nephrology, or others according to the underlying etiology.
6. In anemic patients with ferritin less than 30ucg/L and more than six weeks to an operative or delivery date, oral iron therapy should be instituted.
7. In anemic patients with ferritin less than 30 ucg/L and less than six weeks to an operative or delivery date, IV iron therapy should be instituted.
8.In patients at risk of anemia and ferritin less than 30ucg/L oral iron therapy should be instituted.
9. In patients with anemia and iron restricted erythropoiesis with ferritin greater than 30ucg/L and TSAT less than 20%, IV iron therapy should be instituted.
10. In patients with inadequate response to IV iron or when iron sequestration or inflammation limits the bioavailability of iron, an ESA should be considered on a case-by-case basis.
11. In patients with anemia and evidence of inflammation or renal failure where an ESA is indicated, it should be combined with IV iron.
12. When an ESA is used, concomitant use of thromboembolic prophylaxis should be considered on a case-by-case basis.
13. Anemia should be corrected prior to all elective surgery. Institutions should have guidelines on postponing surgery until anemia is corrected.
14. In patients who develop postoperative or post-hemorrhage related anemia, IV iron is recommended31.
15.The risk of surgical bleeding, urgency of surgery, and type of anticoagulation should be addressed to reduce blood loss. For specific recommendations for individual agents, resources such as Thrombosis Canada or a local perioperative thrombosis expert should be consulted.
16. During hemorrhage, permissive hypotension or deliberately induced hypotension should be considered while balancing the risk of blood loss and preservation of vital organ perfusion.
17. When substantial blood loss is anticipated, acute normovolemic hemodilution should be considered.33,34,35
18. When substantial blood loss is anticipated or encountered, intraoperative cell salvage should be considered.
19. When substantial blood loss is anticipated or encountered, or the patient is involved in trauma or post-partum hemorrhage, antifibrinolytics (tranexamic acid) should be administered.
20. When patients are recovering from anemia, other physiologic parameters should be addressed to reduce oxygen requirements. Hypothermia should be avoided with active warming. Processes that contribute to hospital acquired infections should be minimized, including nasogastric tubes and foley catheters.
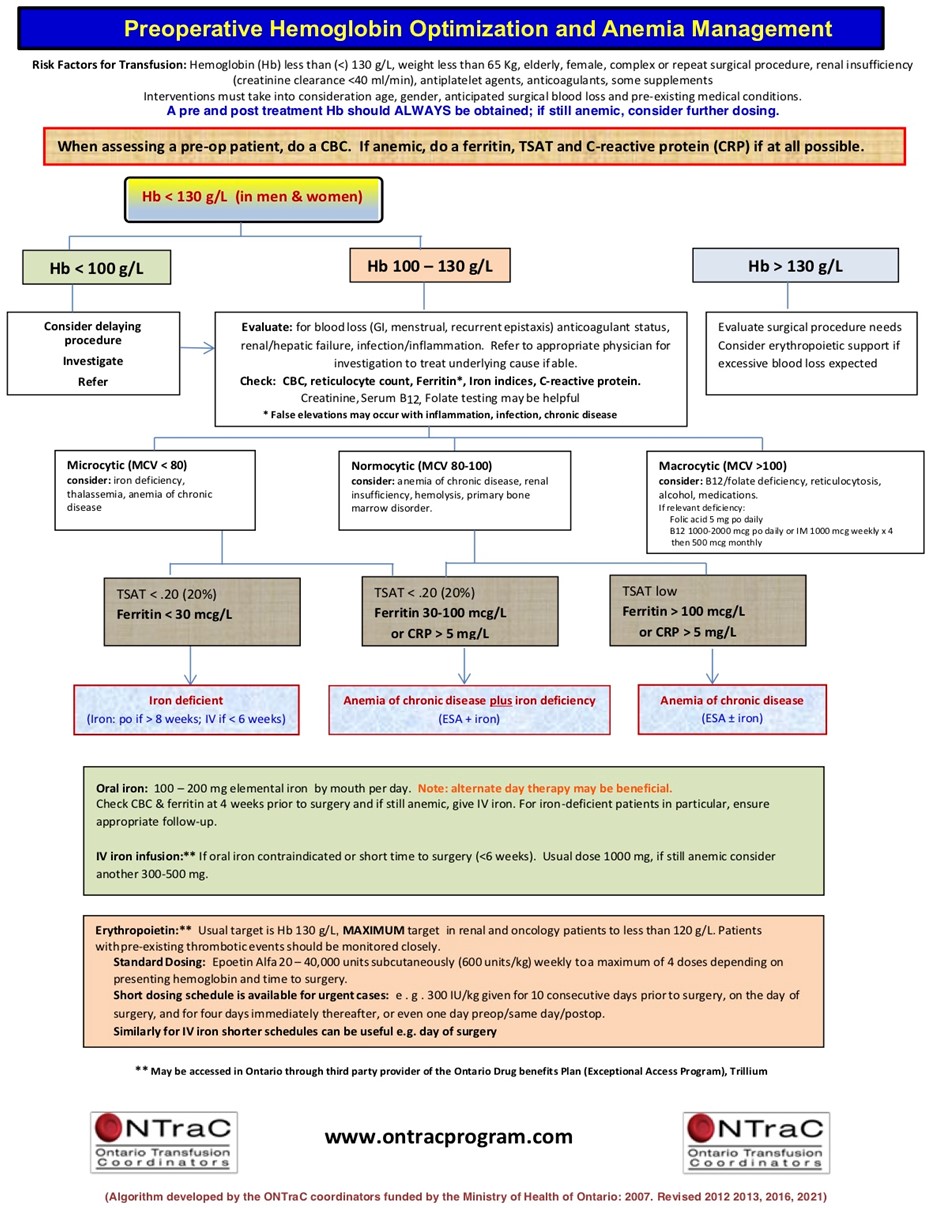
Patients must be placed at the center of care. Improving long term outcomes and reducing morbidity and mortality is necessary to improve the quality of care delivered to Canadians. Implementing multidisciplinary strategies as part of a patient blood management program has the potential to improve outcomes and simultaneously reduce system costs. In the interest of improving care to its citizens, every province should develop a patient blood management program (Figure 1).
Current clinical recommendations already exist and examples are offered from ONTraC24, the American Society of Hematology,32 and the Mayo Clinic2. For visualization purposes, the algorithm from ONTraC is attached.
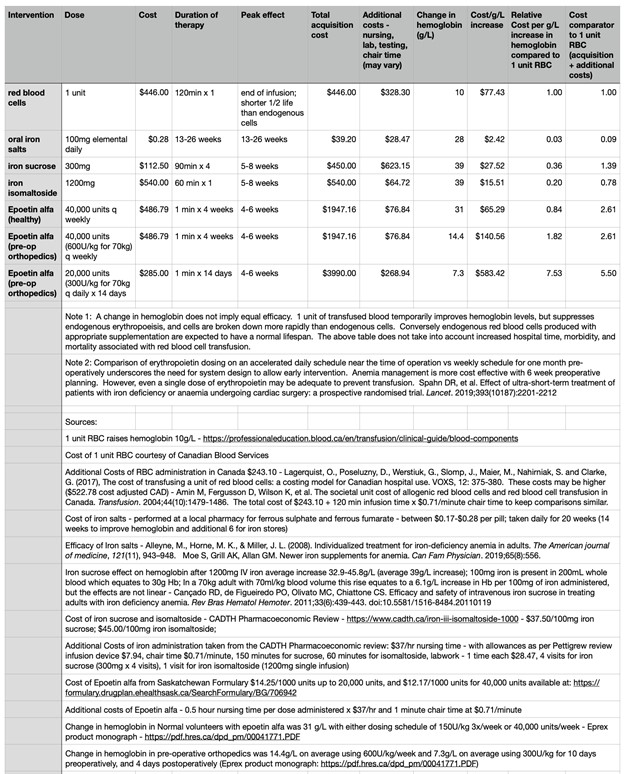
A cost comparison using a single unit of blood as a cost comparison in managing anemia is attached (Table 1). While transfusion of a single unit of blood may raise hemoglobin levels temporarily, the transfused cells are generally destroyed more rapidly than endogenous cells and contribute to inhibiting production of endogenous cells. Transfused blood is not equivalent to endogenous production of blood.
References
1 Society for the Advancement of Blood Management. https://sabm.org accessed January 11, 2021
2 Warner MA, Shore-Lesserson L, Shander A, Patel SY, Perelman SI, Guinn NR. Perioperative Anemia: Prevention, Diagnosis, and Management Throughout the Spectrum of Perioperative Care. Anesth Analg. 2020 May; 130 (5):1364-1380
3 Althoff FC, Neb H, Herrmann E, et al.: Multimodal patient blood management program based on a three-pillar strategy: a systematic review and meta-analysis. Ann Surg 2019; 269:794–804
4 Mueller MM, Van Remoortel H, Meybohm P, et al.: Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019; 321:983–997
5 Farmer, SL et al. A Programmatic Approach to Patient Blood Management - Reducing Transfusions and Improving Patient Outcomes. The Open Anesthesiology Journal. 2015, 9, 6-16
6 Rohde JM, Dimcheff DE, Blumberg N, et al.: Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014; 311:1317–1326
7 Goel R, Patel EU, Cushing MM, et al.: Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg 2018; 153:826–833
8 Whitlock EL, Kim H, Auerbach AD: Harms associated with single unit perioperative transfusion: retrospective population based analysis. BMJ 2015; 350:h3037
9 Pang, QY., An, R. & Liu, HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Onc 17, 7 (2019).
10 Agudelo-Jimenez, R.D., Heatter, J.A. & Cata, J.P. Transfusion Therapy: Is There a Link with Cancer Recurrence?. Curr Anesthesiol Rep 8, 426–438 (2018).
11 John W. Semple, et al; Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 2019; 133 (17): 1840–1853
12 Shander, Bracey, Goodnough, Gross, et al. Patient Blood Management as Standard of Care. Anesthesia & Analgesia: October 2016; 123:4; 1051-1053
13 World Health Organization. Global forum for blood safety: patient blood management concept paper, 2011. https://www. who.int/bloodsafety/events/gfbs_01_pbm_concept_paper.pdf [Last accessed 11 January 2021]
14 World Health Organization. (2021). The urgent need to implement patient blood management: policy brief. World Health Organization. License: CC BY-NC-SA 3.0 IGO
https://apps.who.int/iris/handle/10665/346655. Accessed January 23, 2022
15 Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109(1):55-68. doi:10.1093/bja/aes139
16 Patient Blood Management. Australia National Blood Authority https://www.blood.gov.au/patient-blood-management-pbm Accessed January 11, 2021
17 Canadian Blood Services Professional Education Chapter 9: Blood Administration: https://professionaleducation.blood.ca/en/transfusion/clinical-guide/blood-administration Last accessed January 11, 2020
18 3.1.7 Actual Allocation of Blood Components in Times of Shortages, The National Plan for Management of Shortages of Labile Blood Components. https://www.nacblood.ca/resources/shortages-plan/ Updated March 20, 2020; accessed January 11, 2021
19 Meybohm et al. “Simplified International Recommendations for the Implementation of Patient Blood Management” (SIR4PBM). Perioperative Medicine (2017) 6:5
20 Manzini, P.M.et al. (2018), Patient blood management knowledge and practice among clinicians from seven European university hospitals: a multicentre survey. Vox Sang, 113: 60-71.
21 Choosing Wisely Canada Transfusion Toolkit: https://choosingwiselycanada.org/perspective/transfusion-toolkit/ Accessed January 11, 2021
22 Kei T, Mistry N, Curley G, et al.: Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: a systematic review and meta- analysis. Can J Anaesth 2019; 66:716–731
23 Ontario Transfusion Coordinator website available at: https://www.ontracprogram.com/Public.aspx Accessed January 11, 2021.
24 ONTraC Toolkit available at: https://www.ontracprogram.com/ckupload/files/103/Final%20toolkit%20Aug%2020%202020(1).pdf Accessed January 11, 2021.
25 Pollock, R. F., & Muduma, G. (2017). A budget impact analysis of parenteral iron treatments for iron deficiency anemia in the UK: reduced resource utilization with iron isomaltoside 1000. ClinicoEconomics and outcomes research: CEOR, 9, 475–483
26 Spahn D, et al.: Effect of ultra-short treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomized trial. Lancet 2019; 393:2201–2212
27 Ker K, Edwards P, Perel P, et al.: Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012; 344:e3054
28 Muñoz M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017 Feb; 72 (2):233-247
29 Cho BC, et al. Impact of Preoperative Erythropoietin on Allogeneic Blood Transfusions in Surgical Patients: Results from a Systematic Review and Meta-analysis. Anesth Analg. 2019;128(5):981-992.
30 Derzon JH, et al. Restrictive Transfusion Strategy and Clinical Decision Support Practices for Reducing RBC Transfusion Overuse. Am J Clin Pathol. 2019;152(5):544-557
31 Muñoz, M. et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia, 2018; 73: 1418-1431.
32 Lin, Yulia. Preoperative anemia screening clinics. Hematology: American Society of Hematology Education Program 2019; 2019 (1):570-576
33 Zhou, Xuelong et al. Preoperative Acute Normovolemic Hemodilution for Minimizing Allogeneic Blood Transfusion, Anesthesia & Analgesia: December 2015 – 121:6 - p1443-1455
34 Barile, Luigi MD, et al. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials, Anesthesia & Analgesia: March 2017 - 124:3 - p 743-752
35 L. De Araújo and L. Garcia, "Acute Normovolemic Hemodilution: A Practical Approach," Open Journal of Anesthesiology, Vol. 3 No. 1, 2013, pp. 38-43.
