NAC Recommendations for Management of Dual Inventory of Solvent-Detergent Plasma and Standard Frozen Plasma
Douglas Morrison, MD
Jason Quinn, MD
Katerina Pavenski, MD
Kathryn Webert, MD
Ryan Lett, MD
List of abbreviations
Canadian Blood Services
Frozen Plasma
National Advisory Committee on Blood and Blood Products
Solvent-Detergent Plasma
In July 2022, the Canadian Blood Services (CBS) announced a transition to pathogen-reduced platelet and plasma components as an additional layer of safety to the blood supply system in Canada. The first phase in the implementation of pathogen-reduced plasma is the transition from regular frozen plasma (FP) and solvent-detergent (S/D) treated plasma with a target of 80% of all transfused plasma to be S/D plasma by September 2023. Octaplasma became available from CBS on a non-restricted basis on March 27, 2023.
Currently, there are two plasma products currently available from CBS. Octaplasma, which is a commercially produced solvent-detergent plasma (S/D plasma) product made from pooled plasma donations, is supplied in 200 mL units. FP units produced by CBS vary in size with an average of 289 mLs. As outlined in NAC Recommendations for the use of Solvent Detergent Plasma in Canada, the indications for both plasma products are identical and the products are clinically equivalent. The recommended dose for both products is 10-15 mLs/kg for the correction of abnormal coagulation tests.
The management of a dual inventory of plasma with different unit sizes produces logistical challenges, especially since frozen plasma is usually ordered by the unit. The National Advisory Committee on Blood and Blood Products provides the following suggestions to help with the inventory management of the product:
- As inappropriate dosing of plasma is common, local or regional guidelines for plasma transfusions should provide recommendations for plasma that take the weight of the patient into account to help ensure physicians order the appropriate dose of plasma.
- A standard dose of plasma is 10-15 mLs/ kg when it is given to correct abnormal coagulation test results. This recommended dose should be outlined in local or regional guidelines for plasma transfusions. Including reminders for the appropriate dose for plasma in paper and electronic order sets would further help guide clinicians when they are ordering plasma.
- As S/D plasma will represent the majority of plasma transfused in most hospitals, local and regional guidelines for plasma should consider recommending that plasma be ordered by (i) number of units where the standard unit is 200 mLs and/or (ii) mLs/kg. Including a reminder of the standard 200 mL size for S/D plasma in electronic and paper order sets would also help clinicians order the appropriate plasma dose.
- When filling a request for plasma for correction of an abnormal coagulation test, the transfusion medicine service should have a policy allowing for substitution of FP for S/D plasma (or vice versa) if needed due to inventory issues. Local policies should be developed by transfusion medicine laboratories based on differences in unit volumes, differences in coagulation factor levels and pragmatic considerations. Tables for equivalent weight-based doses of S/D plasma and FP are provided below as examples, though local policy for plasma dosing/substitution will need to be established by transfusion medicine services that include pragmatic considerations.
***Follow local policy for plasma dosing/substitution established by the transfusion medicine service***
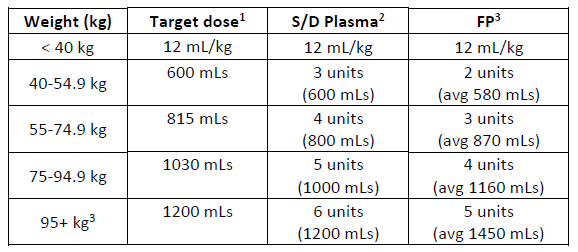
(a) Simple weight-based table for S/D plasma and FP.
(b) Expanded table including dose range for S/D plasma and FP.
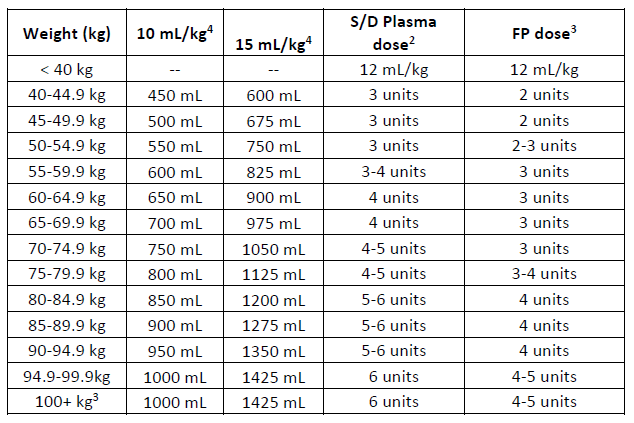
1. Target dose calculated using middle weight x 12.5 mL/kg
2. Number of S/D Plasma units calculated using a unit volume of 200 mLs
3. Number of FP units calculated assuming a mean unit volume of 289 mLs.
4. For weights above 100 kg, the plasma dose is capped using 100kg dose.
5. Dose for 10 mL/kg calculated using upper weight for each category.
6. Dose for 15 mL/kg calculated using lower weight for each category.
